Automated Insulin Delivery with Remote Real-Time Continuous Glucose Monitoring for Hospitalized Patients with Diabetes: A Multicenter, Single-Arm, Feasibility Trial
- PMID: 37578778
- PMCID: PMC10611957
- DOI: 10.1089/dia.2023.0304
Automated Insulin Delivery with Remote Real-Time Continuous Glucose Monitoring for Hospitalized Patients with Diabetes: A Multicenter, Single-Arm, Feasibility Trial
Abstract
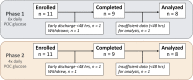
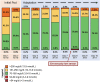
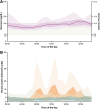
Introduction: Multiple daily injection insulin therapy frequently fails to meet hospital glycemic goals and is prone to hypoglycemia. Automated insulin delivery (AID) with remote glucose monitoring offers a solution to these shortcomings. Research Design and Methods: In a single-arm multicenter pilot trial, we tested the feasibility, safety, and effectiveness of the Omnipod 5 AID System with real-time continuous glucose monitoring (CGM) for up to 10 days in hospitalized patients with insulin-requiring diabetes on nonintensive care unit medical-surgical units. Primary endpoints included the proportion of time in automated mode and percent time-in-range (TIR 70-180 mg/dL) among participants with >48 h of CGM data. Safety endpoints included incidence of severe hypoglycemia and diabetes-related ketoacidosis (DKA). Additional glycemic endpoints, CGM accuracy, and patient satisfaction were also explored. Results: Twenty-two participants were enrolled; 18 used the system for a total of 96 days (mean 5.3 ± 3.1 days per patient), and 16 had sufficient CGM data required for analysis. Median percent time in automated mode was 95% (interquartile range 92%-98%) for the 18 system users, and the 16 participants with >48 h of CGM data achieved an overall TIR of 68% ± 16%, with 0.17% ± 0.3% time <70 mg/dL and 0.06% ± 0.2% time <54 mg/dL. Sensor mean glucose was 167 ± 21 mg/dL. There were no DKA or severe hypoglycemic events. All participants reported satisfaction with the system at study end. Conclusions: The use of AID with a disposable tubeless patch-pump along with remote real-time CGM is feasible in the hospital setting. These results warrant further investigation in randomized trials.
Keywords: Automated insulin delivery; Hybrid closed-loop; Inpatient diabetes.
Conflict of interest statement
G.M.D. has received research support (to Emory University) from Insulet and consulting fees from Medscape, and M.S.H. has received consulting fees from Dexcom, Inc. S.A.B. has received research support (to the University of Virginia) from Insulet, Tandem Diabetes Care, Dexcom, Roche Diagnostics, and Tolerion. T.L. is an employee of Insulet Corporation. R.W.B. reports no personal financial disclosures but reports that his institution has received funding on his behalf as follows: grant funding, study supplies, and consulting fees from Insulet, Tandem Diabetes Care, and Beta Bionics; grant funding and study supplies from Dexcom; grant funding from Bigfoot Biomedical; study supplies from Medtronic, Ascensia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Embecta, Vertex, Hagar, Ypsomed, and Zucara.
R.L. has received consulting fees from Abbott Diabetes Care, Biolinq, Capillary Biomedical, Deep Valley Labs, Morgan Stanley, Gluroo, ProventionBio, PhysioLogic Devices, and Tidepool. He receives research support from NIDDK, JDRF, Insulet, Medtronic, and Tandem. T.T.L. is a fulltime employee of and owns stock in Insulet. B.B. has been on advisory boards for Medtronic Diabetes, Novo Nordisk, Lilly, and received research funding from Insulet, Medtronic, Tandem, JDRF, and the NIDDK. F.J.P. has received research support (to Emory University) from Dexcom, Insulet, Novo Nordisk, and Ideal Medical Technologies, and has received consulting fees from Boehringer Ingelheim, Dexcom, and Medscape. All other authors declare no competing interests.
Figures

References
-
- Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012;97(1):16–38. - PubMed
-
- Committee ADAPP. 16. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl 1):S244–S253. - PubMed
-
- Christensen MB, Gotfredsen A, Nørgaard K. Efficacy of basal-bolus insulin regimens in the inpatient management of non-critically ill patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab Res Rev 2017;33(5):2885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

