A conserved microtubule-binding region in Xanthomonas XopL is indispensable for induced plant cell death reactions
- PMID: 37578981
- PMCID: PMC10449215
- DOI: 10.1371/journal.ppat.1011263
A conserved microtubule-binding region in Xanthomonas XopL is indispensable for induced plant cell death reactions
Abstract
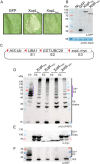
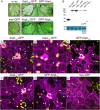
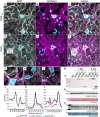
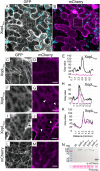
Pathogenic Xanthomonas bacteria cause disease on more than 400 plant species. These Gram-negative bacteria utilize the type III secretion system to inject type III effector proteins (T3Es) directly into the plant cell cytosol where they can manipulate plant pathways to promote virulence. The host range of a given Xanthomonas species is limited, and T3E repertoires are specialized during interactions with specific plant species. Some effectors, however, are retained across most strains, such as Xanthomonas Outer Protein L (XopL). As an 'ancestral' effector, XopL contributes to the virulence of multiple xanthomonads, infecting diverse plant species. XopL homologs harbor a combination of a leucine-rich-repeat (LRR) domain and an XL-box which has E3 ligase activity. Despite similar domain structure there is evidence to suggest that XopL function has diverged, exemplified by the finding that XopLs expressed in plants often display bacterial species-dependent differences in their sub-cellular localization and plant cell death reactions. We found that XopL from X. euvesicatoria (XopLXe) directly associates with plant microtubules (MTs) and causes strong cell death in agroinfection assays in N. benthamiana. Localization of XopLXe homologs from three additional Xanthomonas species, of diverse infection strategy and plant host, revealed that the distantly related X. campestris pv. campestris harbors a XopL (XopLXcc) that fails to localize to MTs and to cause plant cell death. Comparative sequence analyses of MT-binding XopLs and XopLXcc identified a proline-rich-region (PRR)/α-helical region important for MT localization. Functional analyses of XopLXe truncations and amino acid exchanges within the PRR suggest that MT-localized XopL activity is required for plant cell death reactions. This study exemplifies how the study of a T3E within the context of a genus rather than a single species can shed light on how effector localization is linked to biochemical activity.
Copyright: © 2023 Ortmann et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Timilsina S, Potnis N, Newberry EA, Liyanapathiranage P, Iruegas-Bocardo F, White FF, et al.. Xanthomonas diversity, virulence and plant-pathogen interactions. Nat Rev Microbiol. 2020;18(8):415–27. - PubMed
-
- Büttner D, Bonas U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev. 2010;34(2):107–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

