Iron promotes glycolysis to drive colon tumorigenesis
- PMID: 37579983
- PMCID: PMC10530594
- DOI: 10.1016/j.bbadis.2023.166846
Iron promotes glycolysis to drive colon tumorigenesis
Abstract
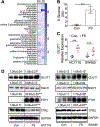
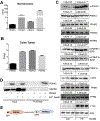
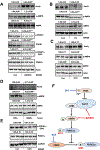
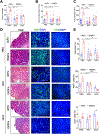
Colorectal cancer (CRC) is the third most common cancer and is also the third leading cause of cancer-related death in the USA. Understanding the mechanisms of growth and progression of CRC is essential to improve treatment. Macronutrients such as glucose are energy source for a cell. Many tumor cells exhibit increased aerobic glycolysis. Increased tissue micronutrient iron levels in both mice and humans are also associated with increased colon tumorigenesis. However, if iron drives colon carcinogenesis via affecting glucose metabolism is still not clear. Here we found the intracellular glucose levels in tumor colonoids were significantly increased after iron treatment. 13C-labeled glucose flux analysis indicated that the levels of several labeled glycolytic products were significantly increased, whereas several tricarboxylic acid cycle intermediates were significantly decreased in colonoids after iron treatment. Mechanistic studies showed that iron upregulated the expression of glucose transporter 1 (GLUT1) and mediated an inhibition of the pyruvate dehydrogenase (PDH) complex function via directly binding with tankyrase and/or pyruvate dehydrogenase kinase (PDHK) 3. Pharmacological inhibition of GLUT1 or PDHK reactivated PDH complex function and reduced high iron diet-enhanced tumor formation. In conclusion, excess iron promotes glycolysis and colon tumor growth at least partly through the inhibition of the PDH complex function.
Keywords: GLUT1; Glucose; Iron; PDH; PDHK3; Tankyrase.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest C.A.L. has received consulting fees from Astellas Pharmaceuticals and Odyssey Therapeutics and is an inventor on patents pertaining to Kras regulated metabolic pathways, redox control pathways in pancreatic cancer, and targeting the GOT1-pathway as a therapeutic approach (US Patent No: 2015126580-A1, 05/07/2015; US Patent No: 20190136238, 05/09/2019; International Patent No: WO2013177426-A2, 04/23/2015).
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

