New biologic (Ab-IPL-IL-17) for IL-17-mediated diseases: identification of the bioactive sequence (nIL-17) for IL-17A/F function
- PMID: 37580108
- PMCID: PMC10579190
- DOI: 10.1136/ard-2023-224479
New biologic (Ab-IPL-IL-17) for IL-17-mediated diseases: identification of the bioactive sequence (nIL-17) for IL-17A/F function
Abstract
Objectives: Interleukin (IL) 17s cytokines are key drivers of inflammation that are functionally dysregulated in several human immune-mediated inflammatory diseases (IMIDs), such as rheumatoid arthritis (RA), psoriasis and inflammatory bowel disease (IBD). Targeting these cytokines has some therapeutic benefits, but issues associated with low therapeutic efficacy and immunogenicity for subgroups of patients or IMIDs reduce their clinical use. Therefore, there is an urgent need to improve the coverage and efficacy of antibodies targeting IL-17A and/or IL-17F and IL-17A/F heterodimer.
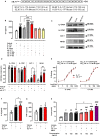
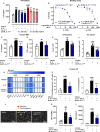
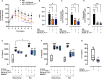
Methods and results: Here, we initially identified a bioactive 20 amino acid IL-17A/F-derived peptide (nIL-17) that mimics the pro-inflammatory actions of the full-length proteins. Subsequently, we generated a novel anti-IL-17 neutralising monoclonal antibody (Ab-IPL-IL-17) capable of effectively reversing the pro-inflammatory, pro-migratory actions of both nIL-17 and IL-17A/F. Importantly, we demonstrated that Ab-IPL-IL-17 has less off-target effects than the current gold-standard biologic, secukinumab. Finally, we compared the therapeutic efficacy of Ab-IPL-IL-17 with reference anti-IL-17 antibodies in preclinical murine models and samples from patients with RA and IBD. We found that Ab-IPL-IL-17 could effectively reduce clinical signs of arthritis and neutralise elevated IL-17 levels in IBD patient serum.
Conclusions: Collectively, our preclinical and in vitro clinical evidence indicates high efficacy and therapeutic potency of Ab-IPL-IL-17, supporting the rationale for large-scale clinical evaluation of Ab-IPL-IL-17 in patients with IMIDs.
Keywords: arthritis, rheumatoid; autoimmune diseases; autoimmunity; inflammation.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ on behalf of EULAR.
Conflict of interest statement
Competing interests: This article has been conducted and written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors ASaviano, FR, FMerlino, RB, PG, MB, AJI and FMaione hold patents for the diagnostic and therapeutic use of nIL-17 and Ab-IPL-IL-17 (IT patent no.: 102022000016722) in autoimmune disease, chronic inflammatory disease and other diseases in which IL-17 producing cells contribute to pathogenesis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

