Neoadjuvant sintilimab in combination with concurrent chemoradiotherapy for locally advanced gastric or gastroesophageal junction adenocarcinoma: a single-arm phase 2 trial
- PMID: 37580320
- PMCID: PMC10425436
- DOI: 10.1038/s41467-023-40480-x
Neoadjuvant sintilimab in combination with concurrent chemoradiotherapy for locally advanced gastric or gastroesophageal junction adenocarcinoma: a single-arm phase 2 trial
Abstract
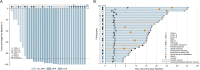
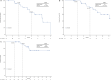
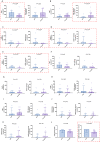
In this multicenter, single-arm phase 2 trial (ChiCTR1900024428), patients with locally advanced gastric/gastroesophageal junction cancers receive one cycle of sintilimab (anti-PD1) and chemotherapy (S-1 and nab-paclitaxel), followed by 5 weeks of concurrent chemoradiotherapy and sintilimab, and another cycle of sintilimab and chemotherapy thereafter. Surgery is preferably scheduled within one to three weeks, and three cycles of adjuvant sintilimab and chemotherapy are administrated. The primary endpoint is the pathological complete response. Our results meet the pre-specified primary endpoint. Thirteen of 34 (38.2%) enrolled patients achieve pathological complete response (95% CI: 22.2-56.4). The secondary objectives include disease-free survival (DFS), major pathological response, R0 resection rate, overall survival (OS), event-free survival (EFS), and safety profile. The median DFS and EFS were 17.0 (95%CI: 11.1-20.9) and 21.1 (95%CI: 14.7-26.1) months, respectively, while the median OS was not reached, and the 1-year OS rate was 92.6% (95%CI: 50.1-99.5%). Seventeen patients (50.0%) have grade ≥3 adverse events during preoperative therapy. In prespecified exploratory biomarker analysis, CD3+ T cells, CD56+ NK cells, and the M1/M1 + M2-like macrophage infiltration at baseline are associated with pathological complete response. Here, we show the promising efficacy and manageable safety profile of sintilimab in combination with concurrent chemoradiotherapy for the perioperative treatment of locally advanced gastric/gastroesophageal junction adenocarcinoma.
© 2023. Springer Nature Limited.
Conflict of interest statement
H.C., S.C., and X.Z. are employees of 3D Medicines Inc. (Shanghai, China). The remaining authors have no conflicts of interest to declare.
Figures

References
-
- Zeng H, et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. 2021;6:e877–e887. - PubMed
-
- Petrelli F, et al. Neoadjuvant chemoradiotherapy or chemotherapy for gastroesophageal junction adenocarcinoma: a systematic review and meta-analysis. Gastric Cancer. 2019;22:245–254. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

