Six-Month Outcomes of Mechanical Thrombectomy for Treating Deep Vein Thrombosis: Analysis from the 500-Patient CLOUT Registry
- PMID: 37580422
- PMCID: PMC10615929
- DOI: 10.1007/s00270-023-03509-8
Six-Month Outcomes of Mechanical Thrombectomy for Treating Deep Vein Thrombosis: Analysis from the 500-Patient CLOUT Registry
Abstract
Purpose: Mechanical thrombectomy for the treatment of deep vein thrombosis (DVT) is being increasingly utilized to reduce symptoms and prevent postthrombotic syndrome (PTS), but more data on clinical outcomes are needed. Mechanical thrombectomy was studied in the ClotTriever Outcomes (CLOUT) registry with 6-month full analysis outcomes reported herein.
Materials and methods: The CLOUT registry is a prospective, all-comer study that enrolled 500 lower extremity DVT patients across 43 US sites treated with mechanical thrombectomy using the ClotTriever System. Core-lab assessed Marder scores and physician-assessed venous patency by duplex ultrasound, PTS assessment using Villalta score, venous symptom severity, pain, and quality of life scores through 6 months were analyzed. Adverse events were identified and independently adjudicated.
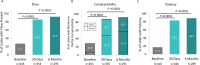
Results: All-cause mortality at 30 days was 0.9%, and 8.6% of subjects experienced a serious adverse event (SAE) within the first 30 days, 1 of which (0.2%) was device related. SAE rethrombosis/residual thrombus incidence was 4.8% at 30 days and 8.0% at 6 months. Between baseline and 6 months, venous flow increased from 27.2% to 92.5% of limbs (P < 0.0001), and venous compressibility improved from 28.0% to 91.8% (P < 0.0001), while median Villalta scores improved from 9.0 at baseline to 1.0 at 6 months (P < 0.0001). Significant improvements in venous symptom severity, pain, and quality of life were also demonstrated. Outcomes from iliofemoral and isolated femoral-popliteal segments showed similar improvements.
Conclusion: Outcomes from the CLOUT study, a large prospective registry for DVT, indicate that mechanical thrombectomy is safe and demonstrates significant improvement in symptoms and health status through 6 months. Level of Evidence 3: Non-randomized controlled cohort/follow-up study.
Keywords: Deep vein thrombosis; Mechanical thrombectomy; Postthrombotic syndrome.
© 2023. The Author(s).
Conflict of interest statement
Abdullah Shaikh, MD, is a speaker for Inari Medical. Mohannad Bisharat, MD, is a consultant for Inari Medical. Nicolas J. Mouawad, MD, MPH, MBA, is a consultant for Inari Medical and Boston Scientific. Eugene Ichinose, MD, is a consultant for Inari Medical, Penumbra, and Angiodynamics, and is a speaker for Pfizer and Bristol Meyers Squib. Steven Abramowitz, MD is a speaker for Inari Medical. Jonathan Lindquist, MD, receives grant support from Inari Medical, Boston Scientific, Sirtex Medical, Philips, is a consultant for Inari Medical, Boston Scientific, Avantec Vascular, Endoshape, Inc., Philips, and Trisalu Life Sciences; and is on the advisory board of Avantec Vascular. Bhavraj Khalsa, MD, is a consultant for Inari Medical. Kalyan Veerina, MD is a speaker for Inari Medical. Thomas S. Maldonado, MD, is a consultant for Inari Medical. Matthew C. Bunte, MD, MS, receives grant support from Inari Medical, and is a consultant for Abbott, Shockwave Medical, and Inari Medical.
Adam Zybulewski, MD, Joseph Paulisin, DO, Adam Raskin, MD, Ezana Azene, MD, PhD, Neil Shah, MD, James Nguyen, MD, Josh Cockrell, MD, Vipul Khetarpaul, MD, Douglas A. Murrey Jr., MS, MD, Edvard Skripochnik, MD, Suman Annambhotla, MD, report no conflicts of interest.
Figures



Comment in
-
Who Holds the Clout? Defining the Role of Mechanical Thrombectomy for Lower Extremity DVT.Cardiovasc Intervent Radiol. 2023 Nov;46(11):1581-1582. doi: 10.1007/s00270-023-03560-5. Epub 2023 Sep 27. Cardiovasc Intervent Radiol. 2023. PMID: 37759087 No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

