Adipose-derived mesenchymal stem cell therapy for reverse bleomycin-induced experimental pulmonary fibrosis
- PMID: 37580529
- PMCID: PMC10425426
- DOI: 10.1038/s41598-023-40531-9
Adipose-derived mesenchymal stem cell therapy for reverse bleomycin-induced experimental pulmonary fibrosis
Abstract
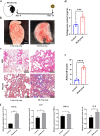
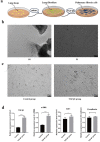
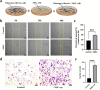
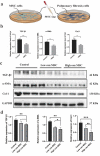
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive respiratory disease. Arguably, the complex interplay between immune cell subsets, coupled with an incomplete understanding of disease pathophysiology, has hindered the development of successful therapies. Despite efforts to understand its pathophysiology and develop effective treatments, IPF remains a fatal disease, necessitating the exploration of new treatment options. Mesenchymal stromal/stem cell (MSC) therapy has shown promise in experimental models of IPF, but further investigation is needed to understand its therapeutic effect. This study aimed to assess the therapeutic effect of adipose-derived mesenchymal stem cells in a bleomycin-induced pulmonary fibrosis model. First, MSC cells were obtained from mice and characterized using flow cytometry and cell differentiation culture methods. Then adult C57BL/6 mice were exposed to endotracheal instillation of bleomycin and concurrently treated with MSCs for reversal models on day 14. Experimental groups were evaluated on days 14, 21, or 28. Additionally, lung fibroblasts challenged with TGF-β1 were treated with MSCs supernatant or MSCs to explore the mechanisms underlying of pulmonary fibrosis reversal. Mesenchymal stem cells were successfully isolated from mouse adipose tissue and characterized based on their differentiation ability and cell phenotype. The presence of MSCs or their supernatant stimulated the proliferation and migration of lung fibrotic cells. MSCs supernatant reduced lung collagen deposition, improved the Ashcroft score and reduced the gene and protein expression of lung fibrosis-related substances. Bleomycin-challenged mice exhibited severe septal thickening and prominent fibrosis, which was effectively reversed by MSCs treatment. MSC supernatant could suppress the TGF-β1/Smad signaling pathway and supernatant promotes fibroblast autophagy. In summary, this study demonstrates that MSCs supernatant treatment is as effective as MSCs in revert the core features of bleomycin-induced pulmonary fibrosis. The current study has demonstrated that MSCs supernatant alleviates the BLM-induced pulmonary fibrosis in vivo. In vitro experiments further reveal that MSC supernatant could suppress the TGF-β1/Smad signaling pathway to inhibit the TGF-β1-induced fibroblast activation, and promotes fibroblast autophagy by Regulating p62 expression. These findings contribute to the growing body of evidence supporting the therapeutic application of MSCs in cell therapy medicine for IPF.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Mishra M, Sindhwani G. Antifibrotics for COVID-19 related lung fibrosis: Agents with benefits? Adv. Respir. Med. 2021;89(2):231–233. - PubMed
-
- Shao D, Liu X, Wu J, et al. Identification of the active compounds and functional mechanisms of Jinshui Huanxian formula in pulmonary fibrosis by integrating serum pharmacochemistry with network pharmacology. Phytomedicine. 2022;102:154177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

