Regional, circuit and network heterogeneity of brain abnormalities in psychiatric disorders
- PMID: 37580620
- PMCID: PMC10471501
- DOI: 10.1038/s41593-023-01404-6
Regional, circuit and network heterogeneity of brain abnormalities in psychiatric disorders
Abstract
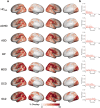
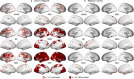
The substantial individual heterogeneity that characterizes people with mental illness is often ignored by classical case-control research, which relies on group mean comparisons. Here we present a comprehensive, multiscale characterization of the heterogeneity of gray matter volume (GMV) differences in 1,294 cases diagnosed with one of six conditions (attention-deficit/hyperactivity disorder, autism spectrum disorder, bipolar disorder, depression, obsessive-compulsive disorder and schizophrenia) and 1,465 matched controls. Normative models indicated that person-specific deviations from population expectations for regional GMV were highly heterogeneous, affecting the same area in <7% of people with the same diagnosis. However, these deviations were embedded within common functional circuits and networks in up to 56% of cases. The salience-ventral attention system was implicated transdiagnostically, with other systems selectively involved in depression, bipolar disorder, schizophrenia and attention-deficit/hyperactivity disorder. Phenotypic differences between cases assigned the same diagnosis may thus arise from the heterogeneous localization of specific regional deviations, whereas phenotypic similarities may be attributable to the dysfunction of common functional circuits and networks.
© 2023. The Author(s).
Conflict of interest statement
K.A. is a scientific advisor to and shareholder in BrainKey Inc., a medical image analysis software company. B.F. has received educational speaking fees from Medice GmbH. C.F.B. is director and shareholder of SBGNeuro Ltd. O.A.A. is a consultant to HealthLytix and received speaker’s honorarium from Lundbeck and Sunovion. N.C. participed in advisory boards and received speaker’s honoraria from Angelini, Esteve, Janssen, Lundbeck, Novartis, Pfizer and Viatris. Furthermore, they have been awarded research grants from the Ministry of Health, Ministry of Science and Innovation (CIBERSAM), and the Strategic Plan for Research and Innovation in Health (PERIS) for the period 2016–2020, as well as from Recercaixa and Marato TV3. M.Y. has received philanthropic donations from the David Winston Turner Endowment Fund, Wilson Foundation, as well as payments in relation to court, expert witness and/or expert review reports. Finally, he has received funding to conduct sponsored Investigator-Initiated trials (including Incannex Healthcare Ltd). These funding sources had no role in the design, management, data analysis, presentation or interpretation and write-up of the data. M.Y. also sits on the Advisory Boards of Centre of The Urban Mental Health, University of Amsterdam; Enosis Therapeutics; and Monash Biomedical Imaging Centre. M.B. has received grant/research support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation; has been a speaker for Abbot, AstraZeneca, Janssen and Janssen, Lundbeck and Merck; and served as a consultant to Allergan, AstraZeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Eisai, Janssen and Janssen, Lundbeck Merck, Pfizer and Servier—all unrelated to this work. M.B. has received grant/research support from National Health and Medical Research Council, Wellcome Trust, Medical Research Future Fund, Victorian Medical Research Acceleration Fund, Centre for Research Excellence CRE, Victorian Government Department of Jobs, Precincts and Regions and Victorian COVID-19 Research Fund. He received honoraria from Springer, Oxford University Press, Cambridge University Press, Allen and Unwin, Lundbeck, Controversias Barcelona, Servier, Medisquire, HealthEd, ANZJP, EPA, Janssen, Medplan, Milken Institute, RANZCP, Abbott India, ASCP, Headspace and Sandoz. The other authors report no conflicts of interest.
Figures












References
-
- Opel N, et al. Cross-disorder analysis of brain structural abnormalities in six major psychiatric disorders: a secondary analysis of mega- and meta-analytical findings from the ENIGMA Consortium. Biol. Psychiatry. 2020;88:678–686. - PubMed

