A phase II dose evaluation pilot feasibility randomized controlled trial of cholecalciferol in critically ill children with vitamin D deficiency (VITdAL-PICU study)
- PMID: 37580663
- PMCID: PMC10424361
- DOI: 10.1186/s12887-023-04205-9
A phase II dose evaluation pilot feasibility randomized controlled trial of cholecalciferol in critically ill children with vitamin D deficiency (VITdAL-PICU study)
Abstract
Background: Vitamin D deficiency (VDD) is highly prevalent in the pediatric intensive care unit (ICU) and associated with worse clinical course. Trials in adult ICU demonstrate rapid restoration of vitamin D status using an enteral loading dose is safe and may improve outcomes. There have been no published trials of rapid normalization of VDD in the pediatric ICU.
Methods: We conducted a multicenter placebo-controlled phase II pilot feasibility randomized clinical trial from 2016 to 2017. We randomized 67 critically ill children with VDD from ICUs in Canada, Chile and Austria using a 2:1 randomization ratio to receive a loading dose of enteral cholecalciferol (10,000 IU/kg, maximum of 400,000 IU) or placebo. Participants, care givers, and outcomes assessors were blinded. The primary objective was to determine whether the loading dose normalized vitamin D status (25(OH)D > 75 nmol/L). Secondary objectives were to evaluate for adverse events and assess the feasibility of a phase III trial.
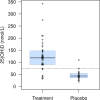
Results: Of 67 randomized participants, one was withdrawn and seven received more than one dose of cholecalciferol before the protocol was amended to a single loading dose, leaving 59 participants in the primary analyses (40 treatment, 19 placebo). Thirty-one/38 (81.6%) participants in the treatment arm achieved a plasma 25(OH)D concentration > 75 nmol/L versus 1/18 (5.6%) the placebo arm. The mean 25(OH)D concentration in the treatment arm was 125.9 nmol/L (SD 63.4). There was no evidence of vitamin D toxicity and no major drug or safety protocol violations. The accrual rate was 3.4 patients/month, supporting feasibility of a larger trial. A day 7 blood sample was collected for 84% of patients. A survey administered to 40 participating families showed that health-related quality of life (HRQL) was the most important outcome for families for the main trial (30, 75%).
Conclusions: A single 10,000 IU/kg dose can rapidly and safely normalize plasma 25(OH)D concentrations in critically ill children with VDD, but with significant variability in 25(OH)D concentrations. We established that a phase III multicentre trial is feasible. Using an outcome collected after hospital discharge (HRQL) will require strategies to minimize loss-to-follow-up.
Clinicaltrials: gov NCT02452762 Registered 25/05/2015.
Keywords: Critical care; Feasibility; Pediatrics; Randomized controlled trial; Vitamin D; Vitamin D deficiency.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
Dr. Khamessan is employed by Euro-Pharm International Canada Inc. Dr. Khamessan and Euro-Pharm International Canada Inc., at the request of the investigative team, developed and prepared the concentrated vitamin D solution used in the study. All other authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Figures

References
-
- Lalgudi Ganesan S, Garros D, Foster J, Di Genova T, Fontela PS, Murthy S. Pediatric critical care capacity in Canada: a national cross-sectional study. medRxiv. 2022:2022.12.07.22283061.
-
- Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192–197. - PubMed
-
- Muranjan MN, Birajdar SB, Shah HR, Sundaraman P, Tullu MS. Psychological consequences in pediatric intensive care unit survivors: the neglected outcome. Indian Pediatr. 2008;45(2):99–103. - PubMed
-
- Ricci MF, Andersen JC, Joffe AR, Watt MJ, Moez EK, Dinu IA, et al. Chronic Neuromotor Disability After Complex Cardiac Surgery in Early Life. Pediatrics. 2015;136(4):e922–e933. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

