Biopharma innovation trends during COVID-19 and beyond: an evidence from global partnerships and fundraising activities, 2011-2022
- PMID: 37580752
- PMCID: PMC10426226
- DOI: 10.1186/s12992-023-00953-6
Biopharma innovation trends during COVID-19 and beyond: an evidence from global partnerships and fundraising activities, 2011-2022
Abstract
Background: Co-development alliances and capital-raising activities are essential supports for biopharmaceutical innovation. During the initial outbreak of the COVID-19, the level of these business activities has increased greatly. Yet the magnitude, direction, and duration of the trend remain ambiguous. Real-time real-world data are needed to inform strategic redirections and industrial policies.
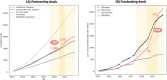
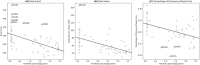
Methods: This observational study aims to characterize trends in global biopharma innovation activities throughout the global pandemic outbreak. Our extensive deal dataset is retrieved from the commercial database GlobalData (12,866 partnership deals and 32,250 fundraising deals announced between 2011 and 2022). We perform Chi-squared tests to examine the changes in qualitative deal attributes during and beyond the outbreak. Our deal-level sample is further aggregated into category-level panel data according to deal characteristics such as therapy area, molecule type, and development phase. We run a series of regressions to examine how the monthly investment amount raised in each category changed with the onset of the pandemic, controlling for the US Federal funds rate.
Results: The temporary surge of partnership and capital-raising activities was associated with the increase in infectious disease-related deals. Academic and government institutions played an increased role in supporting COVID-related co-development partnerships in 2020, and biopharma ventures had been securing more investments in the capital market throughout 2020 and 2021. The partnership and investment boom did not last till the later pandemic in 2022. The most significant and enduring trend was the shifting focus toward discovery-phase investments. Our regression model reveals that the discovery-phase fundraising deals did not suffer from a bounce back in the late pandemic, consistent with a persistent focus on early innovation.
Conclusions: Despite the reduced level of partnership and fundraising activities during 2022, we observe a lasting change in focus toward biopharmaceutical innovation after the pandemic outbreak. Our evidence suggests how entrepreneurs and investors should allocate resources in response to the post-pandemic tight monetary environment. We also suggest the need for policy interventions in financing private/public co-development partnerships and non-COVID-related technologies, to maintain their research capacity and generate breakthroughs when faced with unforeseen diseases.
Keywords: Biopharmaceutical industry; COVID-19; Fundraising; Innovation; Partnership.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
All authors have no relevant financial interests, activities, relationships, or affiliations that may be relevant to the submitted work.
Figures


References
-
- Lamrani HC. Strategic Alliances and Financial Performance: Some Empirical Evidence of Bio-Pharmaceutical Industry. http://tumor.informatics.jax.org/mtbwi/index.do. Accessed 8 Aug 2023.
-
- Crowley WF, Jr, Sherwood L, Salber P, Scheinberg D, Slavkin H, Tilson H, et al. Clinical research in the United States at a crossroads: proposal for a novel public-private partnership to establish a national clinical research enterprise. JAMA. 2004;291(9):1120–1126. doi: 10.1001/jama.291.9.1120. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

