Endovascular treatment for massive haemoptysis due to pulmonary pseudoaneurysm: report of 23 cases
- PMID: 37580779
- PMCID: PMC10426096
- DOI: 10.1186/s13019-023-02346-7
Endovascular treatment for massive haemoptysis due to pulmonary pseudoaneurysm: report of 23 cases
Abstract
Purpose: To evaluate the safety and effectiveness of endovascular treatment for massive haemoptysis caused by pulmonary pseudoaneurysm (PAP).
Methods: The clinical data, imaging data, and endovascular treatment protocol of 23 patients with massive haemoptysis caused by continuous PAP were retrospectively analysed. The success, complications, postoperative recurrence rate, and influence of the treatment on pulmonary artery pressure were also evaluated.
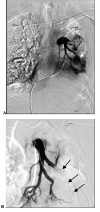
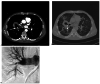
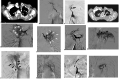
Results: Nineteen patients with a bronchial artery-pulmonary artery (BA-PA) and/or nonbronchial systemic artery-pulmonary artery (NBSA-PA) fistula underwent bronchial artery embolization (BAE) and/or nonbronchial systemic artery embolization (NBSAE) + pulmonary artery embolization (PAE). The pulmonary artery (PA) pressures before and after embolization were 52.11 ± 2.12 (35-69 cmH2O) and 33.58 ± 1.63 (22-44 cmH2O), respectively (P = 0.001). Four patients did not have a BA-PA and/or NBSA-PA fistula. Embolization was performed in two patients with a distal PAP of the pulmonalis lobar arteria. Bare stent-assisted microcoils embolization was performed in the other two patients with a PAP of the main pulmonary lobar arteries. The PA pressures of the four patients before and after treatment were 24.50 ± 1.32 (22-28 cmH2O) and 24.75 ± 1.70 (22-29 cmH2O), respectively (P = 0.850). The technique had a 100% success rate with no serious complications and a postoperative recurrence rate of 30%.
Conclusion: Endovascular treatment is safe and effective for massive haemoptysis caused by PAP. BAE and/or NBSAE can effectively reduce pulmonary hypertension in patients with a BA-PA and/or NBSA-PA fistula.
Keywords: Endovascular treatment; Haemoptysis; Pulmonary artery pseudoaneurysms.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

