Quantitative evaluation of primary lower extremity lymphedema staging using MRI: a preliminary study
- PMID: 37581039
- PMCID: PMC10423363
- DOI: 10.21037/qims-22-795
Quantitative evaluation of primary lower extremity lymphedema staging using MRI: a preliminary study
Abstract
Background: The staging of primary lower extremity lymphedema (LEL) is difficult yet vital in clinical work, and magnetic resonance imaging (MRI) can be used for quantitative assessment of primary LEL due to its high resolution for soft tissues. In this study, we evaluated the value of MRI-based soft tissue area measurements for staging primary LEL.
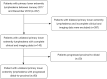
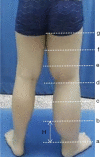
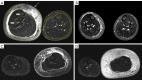
Methods: A total of 90 consecutive patients with clinically diagnosed primary lower limb lymphoedema from January 2017 to December 2019 in Beijing Shijitan Hospital were enrolled retrospectively. Short time inversion recovery (STIR) sequence was applied to measure the total, muscle, bone, and subcutaneous areas in the upper 1/3 level of the bilateral lower calf. The difference between the affected and unaffected calf regarding the subcutaneous area was obtained, and (subcutaneous area)/(bone area) and (subcutaneous area)/(muscle area) were calculated. According to the International Society of Lymphology (ISL) clinical staging standard established in 2020, all patients were divided into stages I, II, and III, accordingly. Statistical analysis was performed to determine the validity of MRI measurements in staging LEL.
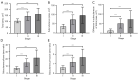
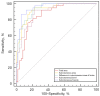
Results: There were 33 patients classified as stage I clinically, 44 patients as stage II, and 13 patients as stage III. There were significant differences in total, subcutaneous, the difference in subcutaneous area of limbs, subcutaneous/bone (S/B), and subcutaneous/muscle (S/M) between stage I and II as well as between stage I and III (P<0.001), but not between stage II and III (P=0.706, 0.329, and 0.229, respectively). A positive correlation was detected between the clinical stage and difference in subcutaneous area of limbs (rho =0.752, P<0.001), S/B (rho =0.747, P<0.001), S/M (rho =0.709, P<0.001), and subcutaneous (rho =0.723, P<0.001). For staging primary LEL, receiver operating characteristic (ROC) curves indicated that the difference in subcutaneous area of limbs had the best discrimination ability among parameters [area under the ROC curve (AUC) =0.950; 95% confidence interval (CI): 0.875-0.987; sensitivity: 95.45%; specificity: 84.85%], followed by S/B (AUC =0.930; 95% CI: 0.848-0.975; sensitivity: 77.27%; specificity: 93.94%) and S/M (AUC =0.895; 95% CI: 0.804-0.953; sensitivity: 77.27%; specificity: 90.91%). The ROC curves indicated that subcutaneous area (AUC =0.927; 95% CI: 0.844-0.974; sensitivity: 84.09%, specificity: 90.91%) and total (AUC =0.852; 95% CI: 0.753-0.923; sensitivity: 70.45%; specificity: 90.91%) also had discrimination ability between stage I and II.
Conclusions: The measurement of the soft tissue area of the calf may be used as an auxiliary method for staging primary LEL. For patients with unilateral primary LEL, the difference in subcutaneous area of limbs could be a specific indicator to distinguish clinical stage I from II.
Keywords: Lymphedema; clinical stage; lower extremity; magnetic resonance imaging (MRI); soft tissue area.
2023 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-795/coif). The authors have no conflicts of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources
