Pyronaridine tetraphosphate is an efficacious antiviral and anti-inflammatory active against multiple highly pathogenic coronaviruses
- PMID: 37581442
- PMCID: PMC10653794
- DOI: 10.1128/mbio.01587-23
Pyronaridine tetraphosphate is an efficacious antiviral and anti-inflammatory active against multiple highly pathogenic coronaviruses
Abstract
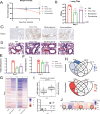
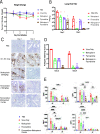
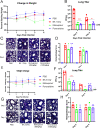
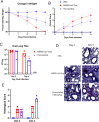
Pyronaridine tetraphosphate is on the WHO Essential Medicine List for its importance as a widely available and safe treatment for malaria. We find that pyronaridine is a highly effective antiviral therapeutic across mouse models using multiple variants of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and the highly pathogenic viruses SARS-CoV-1 and Middle East respiratory syndrome coronavirus responsible for previous coronavirus outbreaks. Additionally, we find that pyronaridine additively combines with current COVID-19 treatments such as nirmatrelvir (protease inhibitor in Paxlovid) and molnupiravir to further inhibit SARS-CoV-2 infections. There are many antiviral compounds that demonstrate efficacy in cellular models, but few that show this level of impact in multiple mouse models and represent a promising therapeutic for the current coronavirus pandemic as well as future outbreaks as well.
Keywords: antiviral agents; coronavirus; preclinical drug studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, Hu Y, Tao Z-W, Tian J-H, Pei Y-Y, Yuan M-L, Zhang Y-L, Dai F-H, Liu Y, Wang Q-M, Zheng J-J, Xu L, Holmes EC, Zhang Y-Z. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579:265–269. doi:10.1038/s41586-020-2008-3 - DOI - PMC - PubMed
-
- Hansen J, Baum A, Pascal KE, Russo V, Giordano S, Wloga E, Fulton BO, Yan Y, Koon K, Patel K, Chung KM, Hermann A, Ullman E, Cruz J, Rafique A, Huang T, Fairhurst J, Libertiny C, Malbec M, Lee W-Y, Welsh R, Farr G, Pennington S, Deshpande D, Cheng J, Watty A, Bouffard P, Babb R, Levenkova N, Chen C, Zhang B, Romero Hernandez A, Saotome K, Zhou Y, Franklin M, Sivapalasingam S, Lye DC, Weston S, Logue J, Haupt R, Frieman M, Chen G, Olson W, Murphy AJ, Stahl N, Yancopoulos GD, Kyratsous CA. 2020. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 369:1010–1014. doi:10.1126/science.abd0827 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

