Rescue of alveolar wall liquid secretion blocks fatal lung injury due to influenza-staphylococcal coinfection
- PMID: 37581936
- PMCID: PMC10541650
- DOI: 10.1172/JCI163402
Rescue of alveolar wall liquid secretion blocks fatal lung injury due to influenza-staphylococcal coinfection
Abstract
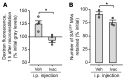
Secondary lung infection by inhaled Staphylococcus aureus (SA) is a common and lethal event for individuals infected with influenza A virus (IAV). How IAV disrupts host defense to promote SA infection in lung alveoli, where fatal lung injury occurs, is not known. We addressed this issue using real-time determinations of alveolar responses to IAV in live, intact, perfused lungs. Our findings show that IAV infection blocked defensive alveolar wall liquid (AWL) secretion and induced airspace liquid absorption, thereby reversing normal alveolar liquid dynamics and inhibiting alveolar clearance of inhaled SA. Loss of AWL secretion resulted from inhibition of the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel in the alveolar epithelium, and airspace liquid absorption was caused by stimulation of the alveolar epithelial Na+ channel (ENaC). Loss of AWL secretion promoted alveolar stabilization of inhaled SA, but rescue of AWL secretion protected against alveolar SA stabilization and fatal SA-induced lung injury in IAV-infected mice. These findings reveal a central role for AWL secretion in alveolar defense against inhaled SA and identify AWL inhibition as a critical mechanism of IAV lung pathogenesis. AWL rescue may represent a new therapeutic approach for IAV-SA coinfection.
Keywords: Epithelial transport of ions and water; Influenza; Innate immunity; Pulmonology.
Figures









References
-
- GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. - DOI - PMC - PubMed

