IgG1-3 MuSK Antibodies Inhibit AChR Cluster Formation, Restored by SHP2 Inhibitor, Despite Normal MuSK, DOK7, or AChR Subunit Phosphorylation
- PMID: 37582613
- PMCID: PMC10427144
- DOI: 10.1212/NXI.0000000000200147
IgG1-3 MuSK Antibodies Inhibit AChR Cluster Formation, Restored by SHP2 Inhibitor, Despite Normal MuSK, DOK7, or AChR Subunit Phosphorylation
Erratum in
-
Missing Full Disclosures.Neurol Neuroimmunol Neuroinflamm. 2025 Jan;12(1):e200342. doi: 10.1212/NXI.0000000000200342. Epub 2024 Oct 30. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 39475708 Free PMC article. No abstract available.
Abstract
Background and objectives: Up to 50% of patients with myasthenia gravis (MG) without acetylcholine receptor antibodies (AChR-Abs) have antibodies to muscle-specific kinase (MuSK). Most MuSK antibodies (MuSK-Abs) are IgG4 and inhibit agrin-induced MuSK phosphorylation, leading to impaired clustering of AChRs at the developing or mature neuromuscular junction. However, IgG1-3 MuSK-Abs also exist in MuSK-MG patients, and their potential mechanisms have not been explored fully.
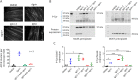
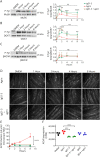
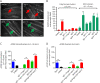
Methods: C2C12 myotubes were exposed to MuSK-MG plasma IgG1-3 or IgG4, with or without purified agrin. MuSK, Downstream of Kinase 7 (DOK7), and βAChR were immunoprecipitated and their phosphorylation levels identified by immunoblotting. Agrin and agrin-independent AChR clusters were measured by immunofluorescence and AChR numbers by binding of 125I-α-bungarotoxin. Transcriptomic analysis was performed on treated myotubes.
Results: IgG1-3 MuSK-Abs impaired AChR clustering without inhibiting agrin-induced MuSK phosphorylation. Moreover, the well-established pathway initiated by MuSK through DOK7, resulting in βAChR phosphorylation, was not impaired by MuSK-IgG1-3 and was agrin-independent. Nevertheless, the AChR clusters did not form, and both the number of AChR microclusters that precede full cluster formation and the myotube surface AChRs were reduced. Transcriptomic analysis did not throw light on the pathways involved. However, the SHP2 inhibitor, NSC-87877, increased the number of microclusters and led to fully formed AChR clusters.
Discussion: MuSK-IgG1-3 is pathogenic but seems to act through a noncanonical pathway. Further studies should throw light on the mechanisms involved at the neuromuscular junction.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
A. Vincent received a proportion of royalties for a patent held by the University of Oxford for use of MuSK for antibody assays, licensed to Athena Diagnostics, that expired in 2021. The other authors report no relevant disclosures. Go to
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
