Dramatic Response to Anti-IL-6 Receptor Therapy in Children With Life-Threatening Myelin Oligodendrocyte Glycoprotein-Associated Disease
- PMID: 37582615
- PMCID: PMC10427143
- DOI: 10.1212/NXI.0000000000200150
Dramatic Response to Anti-IL-6 Receptor Therapy in Children With Life-Threatening Myelin Oligodendrocyte Glycoprotein-Associated Disease
Abstract
Objectives: Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is an immune-mediated neuroinflammatory disorder leading to demyelination of the CNS. Interleukin (IL)-6 receptor blockade is under study in relapsing MOGAD as a preventative strategy, but little is known about the role of such treatment for acute MOGAD attacks.
Methods: We discuss the cases of a 7-year-old boy and a 15-year-old adolescent boy with severe acute CNS demyelination and malignant cerebral edema with early brain herniation associated with clearly positive serum titers of MOG-IgG, whose symptoms were incompletely responsive to standard acute therapies (high-dose steroids, IV immunoglobulins (IVIGs), and therapeutic plasma exchange).
Results: Both boys improved quickly with IL-6 receptor inhibition, administered as tocilizumab. Both patients have experienced remarkable neurologic recovery.
Discussion: We propose that IL-6 receptor therapies might also be considered in acute severe life-threatening presentations of MOGAD.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
J.W. Huh was funded by NIH R01NS110898 and R01NS113945 (but no relevance to this report). B. Banwell serves as a consultant to Novartis, Roche, UCB, Glaxo-Smith Kline, Teva Neuroscience, Sanofi-Genzyme, University of Texas Southwestern, and JRD pharmaceuticals. The other authors have no relevant disclosures. Go to
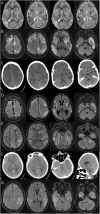
Figures


References
-
- Fainberg N, Silver M, Arena J, et al. . 547: cerebral edema in MOG antibody disease treated with invasive multimodal. Crit Care Med. 2023;51(1):261. doi:10.1097/01.ccm.0000907916.29570.f1 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
