Astrocyte senescence-like response related to peripheral nerve injury-induced neuropathic pain
- PMID: 37582709
- PMCID: PMC10428597
- DOI: 10.1186/s11658-023-00474-5
Astrocyte senescence-like response related to peripheral nerve injury-induced neuropathic pain
Abstract
Background: Peripheral nerve damage causes neuroinflammation, which plays a critical role in establishing and maintaining neuropathic pain (NeP). The mechanisms contributing to neuroinflammation remain poorly elucidated, and pharmacological strategies for NeP are limited. Thus, in this study, we planned to explore the possible link between astrocyte senescence and NeP disorders following chronic sciatic nerve injury.
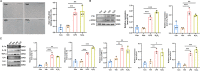
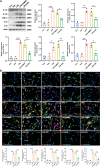
Methods: An NeP animal model was established by inducing chronic constrictive injury (CCI) to the sciatic nerve in adult rats. A senolytic drug combination of dasatinib and quercetin was gavaged daily from the first postoperative day until the end of the study. Paw mechanical withdrawal threshold (PMWT) and paw thermal withdrawal latency (PTWL) were evaluated to assess behaviors in response to pain in the experimental rats. Senescence-associated β-galactosidase staining, western blot analysis, and immunofluorescence were applied to examine the levels of proinflammatory factors and severity of the senescence-like response in the spinal cord. Lipopolysaccharide (LPS) was administered to induce senescence of spinal astrocytes in primary cultures in vitro, to explore the potential impacts of senescence on the secretion of proinflammatory factors. Furthermore, single-cell RNA sequencing (scRNA-seq) was conducted to identify senescence-related molecular responses in spinal astrocytes under neuropathic pain.
Results: Following sciatic nerve CCI, rats exhibited reduced PMWT and PTWL, increased levels of spinal proinflammatory factors, and an enhanced degree of senescence in spinal astrocytes. Treatment with dasatinib and quercetin effectively attenuated spinal neuroinflammation and mitigated the hypersensitivities of the rats subjected to sciatic nerve CCI. Mechanistically, the dasatinib-quercetin combination reversed senescence in LPS-stimulated primary cultured astrocytes and decreased the levels of proinflammatory factors. The scRNA-seq data revealed four potential senescence-related genes in the spinal astrocyte population, and the expression of clusterin (CLU) protein was validated via in vitro experiments.
Conclusion: The findings indicate the potential role of astrocyte senescence in neuroinflammation following peripheral nerve injury, and suggest that targeting CLU activation in astrocytes might provide a novel therapeutic strategy to treat NeP.
Keywords: Astrocyte; Neuroinflammation; Neuropathic pain; Peripheral nerve injury; Senescence.
© 2023. University of Wroclav.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

