NeuroD4 converts glioblastoma cells into neuron-like cells through the SLC7A11-GSH-GPX4 antioxidant axis
- PMID: 37582760
- PMCID: PMC10427652
- DOI: 10.1038/s41420-023-01595-8
NeuroD4 converts glioblastoma cells into neuron-like cells through the SLC7A11-GSH-GPX4 antioxidant axis
Abstract
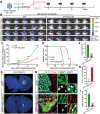
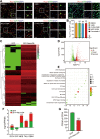
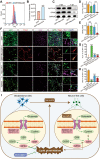
Cell fate and proliferation ability can be transformed through reprogramming technology. Reprogramming glioblastoma cells into neuron-like cells holds great promise for glioblastoma treatment, as it induces their terminal differentiation. NeuroD4 (Neuronal Differentiation 4) is a crucial transcription factor in neuronal development and has the potential to convert astrocytes into functional neurons. In this study, we exclusively employed NeuroD4 to reprogram glioblastoma cells into neuron-like cells. In vivo, the reprogrammed glioblastoma cells demonstrated terminal differentiation, inhibited proliferation, and exited the cell cycle. Additionally, NeuroD4 virus-infected xenografts exhibited smaller sizes compared to the GFP group, and tumor-bearing mice in the GFP+NeuroD4 group experienced prolonged survival. Mechanistically, NeuroD4 overexpression significantly reduced the expression of SLC7A11 and Glutathione peroxidase 4 (GPX4). The ferroptosis inhibitor ferrostatin-1 effectively blocked the NeuroD4-mediated process of neuron reprogramming in glioblastoma. To summarize, our study demonstrates that NeuroD4 overexpression can reprogram glioblastoma cells into neuron-like cells through the SLC7A11-GSH-GPX4 signaling pathway, thus offering a potential novel therapeutic approach for glioblastoma.
© 2023. Cell Death Differentiation Association (ADMC).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Ibuprofen induces ferroptosis of glioblastoma cells via downregulation of nuclear factor erythroid 2-related factor 2 signaling pathway.Anticancer Drugs. 2020 Jan;31(1):27-34. doi: 10.1097/CAD.0000000000000825. Anticancer Drugs. 2020. PMID: 31490283
-
Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification.Kidney Int. 2022 Dec;102(6):1259-1275. doi: 10.1016/j.kint.2022.07.034. Epub 2022 Sep 3. Kidney Int. 2022. PMID: 36063875
-
RSL3 Drives Ferroptosis through NF-κB Pathway Activation and GPX4 Depletion in Glioblastoma.Oxid Med Cell Longev. 2021 Dec 26;2021:2915019. doi: 10.1155/2021/2915019. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34987700 Free PMC article.
-
Astragaloside IV regulates the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to inhibit PM2.5-mediated lung injury in mice.Int Immunopharmacol. 2022 Nov;112:109186. doi: 10.1016/j.intimp.2022.109186. Epub 2022 Sep 15. Int Immunopharmacol. 2022. PMID: 36115280 Review.
-
System Xc -/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy.Front Pharmacol. 2022 Aug 29;13:910292. doi: 10.3389/fphar.2022.910292. eCollection 2022. Front Pharmacol. 2022. PMID: 36105219 Free PMC article. Review.
Cited by
-
GNAO1 overexpression promotes neural differentiation of glioma stem-like cells and reduces tumorigenicity through TRIM21/CREB/HES1 axis.Oncogene. 2025 Mar;44(7):450-461. doi: 10.1038/s41388-024-03234-7. Epub 2024 Nov 23. Oncogene. 2025. PMID: 39580518
-
Molecular biology of the deadliest cancer - glioblastoma: what do we know?Front Immunol. 2025 Mar 21;16:1530305. doi: 10.3389/fimmu.2025.1530305. eCollection 2025. Front Immunol. 2025. PMID: 40191211 Free PMC article. Review.
-
Continuous Theta Burst Stimulation Inhibits Oxidative Stress-Induced Inflammation and Autophagy in Hippocampal Neurons by Activating Glutathione Synthesis Pathway, Improving Cognitive Impairment in Sleep-Deprived Mice.Neuromolecular Med. 2024 Oct 10;26(1):40. doi: 10.1007/s12017-024-08807-z. Neuromolecular Med. 2024. PMID: 39388015
References
-
- Chen J, Han P, Dahiya S. Glioblastoma: changing concepts in the WHO CNS5 classification. Indian J Pathol Microbiol. 2022;65:S24–32. - PubMed
-
- Komori T. Grading of adult diffuse gliomas according to the 2021 WHO classification of tumors of the central nervous system. Lab Invest. 2022;102:126–33. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

