A sex-specific thermogenic neurocircuit induced by predator smell recruiting cholecystokinin neurons in the dorsomedial hypothalamus
- PMID: 37582805
- PMCID: PMC10427624
- DOI: 10.1038/s41467-023-40484-7
A sex-specific thermogenic neurocircuit induced by predator smell recruiting cholecystokinin neurons in the dorsomedial hypothalamus
Abstract
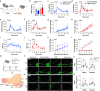
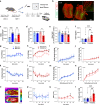
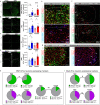
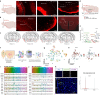
Olfactory cues are vital for prey animals like rodents to perceive and evade predators. Stress-induced hyperthermia, via brown adipose tissue (BAT) thermogenesis, boosts physical performance and facilitates escape. However, many aspects of this response, including thermogenic control and sex-specific effects, remain enigmatic. Our study unveils that the predator odor trimethylthiazoline (TMT) elicits BAT thermogenesis, suppresses feeding, and drives glucocorticoid release in female mice. Chemogenetic stimulation of olfactory bulb (OB) mitral cells recapitulates the thermogenic output of this response and associated stress hormone corticosterone release in female mice. Neuronal projections from OB to medial amygdala (MeA) and dorsomedial hypothalamus (DMH) exhibit female-specific cFos activity toward odors. Cell sorting and single-cell RNA-sequencing of DMH identify cholecystokinin (CCK)-expressing neurons as recipients of predator odor cues. Chemogenetic manipulation and neuronal silencing of DMHCCK neurons further implicate these neurons in the propagation of predator odor-associated thermogenesis and food intake suppression, highlighting their role in female stress-induced hyperthermia.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Rabasa C, Dickson SL. Impact of stress on metabolism and energy balance. Curr Opin. Behav. Sci. 2016;9:71–77.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

