Closing the loop between brain and electrical stimulation: towards precision neuromodulation treatments
- PMID: 37582922
- PMCID: PMC10427701
- DOI: 10.1038/s41398-023-02565-5
Closing the loop between brain and electrical stimulation: towards precision neuromodulation treatments
Abstract
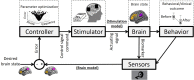
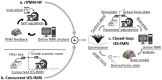
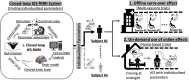
One of the most critical challenges in using noninvasive brain stimulation (NIBS) techniques for the treatment of psychiatric and neurologic disorders is inter- and intra-individual variability in response to NIBS. Response variations in previous findings suggest that the one-size-fits-all approach does not seem the most appropriate option for enhancing stimulation outcomes. While there is a growing body of evidence for the feasibility and effectiveness of individualized NIBS approaches, the optimal way to achieve this is yet to be determined. Transcranial electrical stimulation (tES) is one of the NIBS techniques showing promising results in modulating treatment outcomes in several psychiatric and neurologic disorders, but it faces the same challenge for individual optimization. With new computational and methodological advances, tES can be integrated with real-time functional magnetic resonance imaging (rtfMRI) to establish closed-loop tES-fMRI for individually optimized neuromodulation. Closed-loop tES-fMRI systems aim to optimize stimulation parameters based on minimizing differences between the model of the current brain state and the desired value to maximize the expected clinical outcome. The methodological space to optimize closed-loop tES fMRI for clinical applications includes (1) stimulation vs. data acquisition timing, (2) fMRI context (task-based or resting-state), (3) inherent brain oscillations, (4) dose-response function, (5) brain target trait and state and (6) optimization algorithm. Closed-loop tES-fMRI technology has several advantages over non-individualized or open-loop systems to reshape the future of neuromodulation with objective optimization in a clinically relevant context such as drug cue reactivity for substance use disorder considering both inter and intra-individual variations. Using multi-level brain and behavior measures as input and desired outcomes to individualize stimulation parameters provides a framework for designing personalized tES protocols in precision psychiatry.
© 2023. Springer Nature Limited.
Conflict of interest statement
The City University of New York holds patents on brain stimulation with MB as an inventor. MB has equity in Soterix Medical Inc. MB consults, received grants, assigned inventions, and/or serves on the SAB of Boston Scientific, GlaxoSmithKline, Biovisics, Mecta, Lumenis, Halo Neuroscience, Google-X, i-Lumen, Humm, Allergan (Abbvie). This work has been supported in part by The William K. Warren Foundation and the National Institute of General Medical Sciences Center Grant Award Number (1P20GM121312) and the National Institute on Drug Abuse (U01DA050989). MP is an advisor to Spring Care, Inc., a behavioral health startup, he has received royalties for an article about methamphetamine in UpToDate. MP has a consulting agreement with and receives compensation from F. Hoffmann-La Roche Ltd. This study is also supported by funds from Laureate Institute for Brain Research (LIBR), Tulsa, OK, and Medical Discovery Team on Addiction (MDTA), University of Minnesota, Minneapolis, MN and Brain and Behavior Foundation (NARSAD Young Investigator Award #27305) to HE. MAN is member of the Scientific Advisory Boards of Neuroelectrics, and Precisis. All other authors reported no competing interests.
Figures


References
-
- Brake K, Gumireddy A, Tiwari A, Chauhan H, Kumari D. In vivo studies for drug development via oral delivery: challenges, animal models and techniques. Pharm Anal Acta. 2017;8:560.
-
- Rudin M, Beckmann N, Mir A, Sauter A. In vivo magnetic resonance imaging and spectroscopy in pharmacological research: assessment of morphological, physiological and metabolic effects of drugs. Eur J Pharm Sci. 1995;3:255–64..
-
- Beckmann N, Kneuer R, Gremlich HU, Karmouty‐Quintana H, Blé FX, Müller M. In vivo mouse imaging and spectroscopy in drug discovery. NMR in biomedicine: an international journal devoted to the development and application of magnetic resonance. vivo. 2007;20:154–85. - PubMed
-
- Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4:35–44. - PubMed
-
- Whitebread S, Hamon J, Bojanic D, Urban L. Keynote review: in vitro safety pharmacology profiling: an essential tool for successful drug development. Drug Discov today. 2005;10:1421–33. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

