Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results
- PMID: 37582952
- PMCID: PMC10504075
- DOI: 10.1038/s41591-023-02528-9
Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results
Abstract
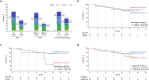
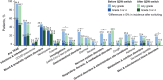
Elranatamab is a humanized B-cell maturation antigen (BCMA)-CD3 bispecific antibody. In the ongoing phase 2 MagnetisMM-3 trial, patients with relapsed or refractory multiple myeloma received subcutaneous elranatamab once weekly after two step-up priming doses. After six cycles, persistent responders switched to biweekly dosing. Results from cohort A, which enrolled patients without prior BCMA-directed therapy (n = 123) are reported. The primary endpoint of confirmed objective response rate (ORR) by blinded independent central review was met with an ORR of 61.0% (75/123); 35.0% ≥complete response. Fifty responders switched to biweekly dosing, and 40 (80.0%) improved or maintained their response for ≥6 months. With a median follow-up of 14.7 months, median duration of response, progression-free survival and overall survival (secondary endpoints) have not been reached. Fifteen-month rates were 71.5%, 50.9% and 56.7%, respectively. Common adverse events (any grade; grade 3-4) included infections (69.9%, 39.8%), cytokine release syndrome (57.7%, 0%), anemia (48.8%, 37.4%), and neutropenia (48.8%, 48.8%). With biweekly dosing, grade 3-4 adverse events decreased from 58.6% to 46.6%. Elranatamab induced deep and durable responses with a manageable safety profile. Switching to biweekly dosing may improve long-term safety without compromising efficacy. ClinicalTrials.gov identifier: NCT04649359 .
© 2023. The Author(s).
Conflict of interest statement
A.M.L. reports consulting or advisory roles for Pfizer, Trillium Therapeutics and Arcellx; personal fees from ITeos Therapeutics, Janssen, Legend Biotech, Pfizer, Sanofi and Trillium Therapeutics; institutional research support from Bristol-Myers Squibb, Genentech, Janssen Oncology, Pfizer, Sanofi, Trillium Therapeutics and patents, royalties and/or other intellectual property interests with Serametrix. M.H.T. reports consulting or advisory roles for Janssen. G.K., Y.J., A.E.G. and D.A.S. have no competing interests to report. B.A. reports consulting or advisory roles for Amgen, Celgene, Janssen-Cilag and Sanofi; personal fees from Celgene, Janssen-Cilag, Sanofi and Takeda; research support from Janssen-Cilag and travel and accommodations expenses paid for by Amgen, Celgene, Janssen-Cilag and Takeda. N.J.B. reports consulting or advisory roles for Amgen, Celgene, Janssen, Karyopharm Therapeutics, Pfizer, Sanofi and Takeda; personal fees from AbbVie, Amgen, Celgene, Genentech/Roche, GSK, Janssen, Karyopharm Therapeutics, Sanofi and Takeda and patents, royalties, and/or other intellectual property interests with Celgene and Janssen. H.M.P. reports consulting or advisory roles for Bristo-Myers Squibb, GSK, Janssen, Takeda and Sanofi and research support from AbbVie and Bristol-Myers Squibb. R.N. reports consulting or advisory roles for Bristol-Myers Squibb, GSK, Janssen, Karyopharm Therapeutics and Takeda and research support from Bristol-Myers Squibb, GSK, Janssen, Karyopharm Therapeutics and Takeda. P.R.O. reports consulting or advisory roles for AbbVie, Bristol-Myers Squibb, GSK, Janssen, Pfizer and Sanofi; personal fees from AbbVie, Celgene, GSK, H3 Biomedicine, Janssen, Pfizer and Sanofi; travel and accommodations expenses paid for by Pfizer and speaker’s bureau roles with Bristol-Myers Squibb, GSK, Janssen and Sanofi. J.M.L. reports consulting or advisory roles for Bristol-Myers Squibb, Janssen Oncology and Novartis; institutional research support from Astellas Pharma and Bristol-Myers Squibb and speaker’s bureau roles with Bristol-Myers Squibb, Janssen-Cilag and Roche. C.T. reports consulting or advisory roles for AbbVie, Amgen, Celgene, GSK, Janssen, Novartis and Takeda; personal fees from AbbVie, Amgen, Celgene, GSK, Janssen, Novartis, Sanofi and Takeda; research support from AbbVie, GSK and Sanofi and travel and accommodations expenses paid for by Pfizer and Janssen. H.Q. reports consulting or advisory roles for Amgen, Antengene, Bristol-Myers Squibb/Celgene, Celgene, CSL Behring, GSK, Janssen-Cilag, Karyopharm Therapeutics, Pfizer, Roche and Sanofi and research support from Amgen, Bristol-Myers Squibb/Celgene, Celgene, GSK, Karyopharm Therapeutics and Sanofi. J.D. reports a consulting or advisory role for Novartis and travel and accommodations expenses paid for by AstraZeneca Spain. H.Y. reports research support from Astellas Pharma. A.K.N. reports consulting or advisory roles for Adaptive Biotechnologies, Amgen, BeyondSpring Pharmaceuticals, Bristol-Myers Squibb, Cellectar, Genzyme, GSK, Janssen Oncology, Karyopharm Therapeutics, Oncopeptides, ONK Therapeutics, Pfizer, Secura Bio and Takeda; personal fees from Adaptive Biotechnologies, Amgen, BeyondSpring Pharmaceuticals, Bristol-Myers Squibb/Celgene, Cellectar, Genzyme, GSK, Janssen Oncology, Karyopharm Therapeutics, Oncopeptides, ONK Therapeutics, Pfizer, Secura Bio and Takeda; institutional research support from Amgen, Arch Oncology, Bristol-Myers Squibb/Celgene, Cellectar, GSK, Janssen Oncology, Pfizer and Takeda and travel and accommodations expenses paid for by GSK. S.M. reports consulting or advisory roles for AbbVie, Adaptive Biotechnologies, Amgen, Bristol-Myers Squibb/Celgene, GSK, Janssen, Regeneron, Roche, Sanofi and Takeda. N.R. reports consulting or advisory roles for Amgen, Bristol-Myers Squibb, Celgene, GSK, Janssen, Merck and Takeda; personal fees from Medscape and Research To Practice and research support from 2Seventy Bio. S.I. reports consulting or advisory roles for Janssen, Sanofi, Takeda, Pfizer, Novartis, Bristol-Myers Squibb and AbbVie; personal fees from Bristol-Myers Squibb, Celgene, Janssen, Ono Pharmaceutical, Pfizer, Sanofi, and Takeda and research support from AbbVie, Amgen, Bristol-Myers Squibb, Caelum Biosciences, Celgene, Daiichi Sankyo Pharmaceutical, Janssen, Ono Pharmaceutical, Otsuka, Sanofi, Shionogi and Takeda. M.S.R. reports consulting or advisory roles for Amgen, Bristol-Myers Squibb, GSK, Janssen, Oncopeptides, Pfizer and Sanofi; personal fees from AbbVie, Bristol-Myers Squibb, GSK and Takeda and research support from Bristol-Myers Squibb, Janssen, Novartis and Sanofi. E.S. reports consulting or advisory roles for Shattuck Labs and Sanofi; personal fees from AbbVie, Janssen and travel and accommodations expenses paid for by Janssen. E.L., S.T.S., U.C., M.E., A.C. and A.V. report employment and stock ownership at Pfizer. M.M. reports consulting or advisory roles for Adaptive Biotechnologies, GSK, Jazz Pharmaceuticals, MaaT Pharma, Novartis, Sanofi and Xenikos; personal fees from Amgen, Astellas, Bristol-Myers Squibb, Celgene, Pfizer, Stemline-Menarini and Takeda and speaker’s bureau roles with Janssen, Jazz Pharmaceuticals and Sanofi.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

