Clinical and Ultrasound Evaluation of Skin Quality After Subdermal Injection of Two Non-Crosslinked Hyaluronic Acid-Based Fillers
- PMID: 37583485
- PMCID: PMC10424680
- DOI: 10.2147/CCID.S402409
Clinical and Ultrasound Evaluation of Skin Quality After Subdermal Injection of Two Non-Crosslinked Hyaluronic Acid-Based Fillers
Abstract
Introduction: Nowadays patients want to get an immediate result from skin rejuvenation techniques without sign of injections and consequent limitations in social life. Therefore, the least traumatic, more effective, and longer lasting treatment approach for skin quality improvement should be favored.
Purpose: Assess skin quality outcomes by clinical examination and self-reporting in patients treated with two non-crosslinked hyaluronic acid (HA) products, injected by cannula. Investigate the skin thickness and the longevity of dermal fillers in soft tissues by ultrasound examination.
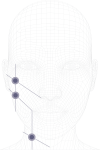
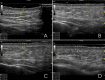
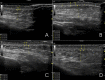
Patients and methods: Fifteen female patients (mean age 41 years) were selected for injection with two non-crosslinked HA products (one for each hemiface and hemi neck). Subdermal injections were performed bilaterally and the retrograde linear fanning technique with a 25G 50 mm cannula from three entry points was used. An ultrasound examination of the skin layers thickness was carried out before the procedure and every 6-7 days up to three weeks, when patients skin quality improvement was assessed by GAIS (Global Aesthetic Improvement Scale) and patients asked about their satisfaction level.
Results: On the right hemiface, the use of the non-crosslinked HA-product with lidocaine was not associated with pain in the sites of injection. On both face sides, the signs of bruising or edema were minor and not associated with downtime or social life limitation after the procedure. After three weeks, despite both injected products could not be detected by ultrasound technique, signs of skin stimulation and skin layers hydration were still observed: The dermis became thicker on both hemifaces while the epidermis became thinner but showed more pronounced radiance and densification effect on the right hemiface.
Conclusion: Subdermal injections of non-crosslinked HA "skin boosters" could be a good option for minimal traumatic and effective 3-week lasting skin quality improvement.
Keywords: HA; lidocaine; skin booster; skin texture; skin tone; tissue longevity; ultrasound scan.
© 2023 Bezpalko and Filipskiy.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Gu Y, Han J, Jiang C, Zhang Y. Biomarkers, oxidative stress, and autophagy in skin aging. Ageing Res Rev. 2020;59:101036. - PubMed
-
- Tobin DJ. Introduction to skin aging. J Tissue Viability. 2017;26(1):37–46. - PubMed
-
- Facial Rejuvenation Market size was estimated to be US$ 19 billion in 2019: insight org/10.1021/cr050247k SLICE September 22, 2020. Available from: https://www.globenewswire.com/newsrelease/2020/09/22/2097541/0/en/Facial.... Accessed July 19, 2023.
Publication types
LinkOut - more resources
Full Text Sources

