An effective method for establishing a regeneration and genetic transformation system for Actinidia arguta
- PMID: 37583592
- PMCID: PMC10425222
- DOI: 10.3389/fpls.2023.1204267
An effective method for establishing a regeneration and genetic transformation system for Actinidia arguta
Abstract
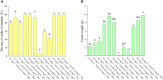
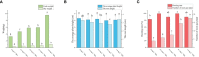
The all-red A. arguta (Actinidia arguta) is an anthocyanin-rich and excellent hardy fruit. Many studies have focused on the green-fleshed A. arguta, and fewer studies have been conducted on the all-red A. arguta. Here we reported a regeneration and Agrobacterium-mediated transformation protocol by using leaves of all-red A. arguta as explants. Aseptic seedling leaves of A. arguta were used as callus-inducing materials. MS medium supplemented with 0.3 mg·L-1 2,4-D and 1.0 mg·L-1 BA was the optimal medium for callus induction of leaves, and medium supplemented with 3 mg·L-1 tZ and 0.5 mg·L-1 IAA was optimal for adventitious shoot regeneration. The best proliferation medium for adventitious buds was MS + 1.0 mg·L-1 BA + 0.3 mg·L-1 NAA. The best rooting medium was 1/2MS + 0.7 mg·L-1 IBA with a 100% rooting rate. For the red flesh hardy kiwi variety 'Purpurna Saduwa' (A. arguta var. purpurea), leaves are receptors for Agrobacterium (EHA105)-mediated transformation. The orthogonal experiment was used for the optimization of each genetic transformation parameter and the genetic transformation of the leaves was 21% under optimal conditions. Our study provides technical parameters for applying genetic resources and molecular breeding of kiwifruit with red flesh.
Keywords: Actinidia arguta; genetic transformation; plant growth regulators; regeneration system; tissue cultures.
Copyright © 2023 Yao, Kong, Lei, Zhao, Tang, Zhou, Lin, Zhang, Wang, He, Li, Chen, Luo, Wang, Tang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



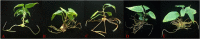





References
-
- Abdullah M., Sliwinska E., Góralski G., Tuleja M., Widyna P., Popielarska-Konieczna M. (2021). Effect of medium composition, genotype and age of explant on the regeneration of hexaploid plants from endosperm culture of tetraploid kiwiberry (Actinidia arguta). Plant Cell Tissue Organ Culture 147, 569–582. doi: 10.1007/s11240-021-02149-5 - DOI
-
- Abraham J., Cheruvathur M. K., Mani B., Thomas D. T., Dennis T. (2010). A rapid in vitro multiplication system for commercial propagation of pharmaceutically important cyclea peltata (Lam) hook & thoms. based on enhanced axillary branching. Ind. Crops Products 31, 92–98. doi: 10.1016/j.indcrop.2009.09.011 - DOI
-
- Ahmed M., Khan N., Hafiz I. A., Abbasi N. (2015). Effect of various factors on the efficiency of agrobacterium-mediated transformation of grepe (Vitis vinifera l.). Vegetos 28 (5), 171–178. doi: 10.5958/2229-4473.2015.00024.5 - DOI
-
- Asakura I., Hoshino Y. (2017). Endosperm-derived triploid plant regeneration in diploid actinidia kolomikta, a cold-hardy kiwifruit relative. Scientia Horticulturae 219, 53–59. doi: 10.1016/j.scienta.2017.02.045 - DOI
-
- Chen J., Wang L., Chen J., Huang J., Liu F., Guo R., et al. (2018). Agrobacterium tumefaciens-mediated transformation system for the important medicinal plant dendrobium catenatum lindl. In Vitro Cell. Dev. Biology-Plant 54 (3), 228–239. doi: 10.1007/s11627-018-9903-4 - DOI
LinkOut - more resources
Full Text Sources

