The decoupling between hemodynamic parameters and neural activity implies a complex origin of spontaneous brain oscillations
- PMID: 37583895
- PMCID: PMC10423917
- DOI: 10.3389/fncom.2023.1214793
The decoupling between hemodynamic parameters and neural activity implies a complex origin of spontaneous brain oscillations
Abstract
Introduction: Spontaneous low-frequency oscillations play a key role in brain activity. However, the underlying mechanism and origin of low-frequency oscillations remain under debate.
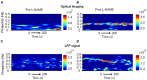
Methods: Optical imaging and an electrophysiological recording system were combined to investigate spontaneous oscillations in the hemodynamic parameters and neuronal activity of awake and anesthetized mice after Nω-nitro-L-arginine methyl ester (L-NAME) administration.
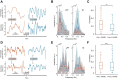
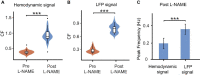
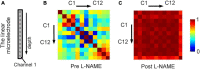
Results: The spectrum of local field potential (LFP) signals was significantly changed by L-NAME, which was further corroborated by the increase in energy and spatial synchronization. The important finding was that L-NAME triggered regular oscillations in both LFP signals and hemodynamic signals. Notably, the frequency peak of hemodynamic signals can be different from that of LFP oscillations in awake mice.
Discussion: A model of the neurovascular system was proposed to interpret this mismatch of peak frequencies, supporting the view that spontaneous low-frequency oscillations arise from multiple sources.
Keywords: local field potential; nitric oxide synthase inhibitor; optical imaging; signal decoupling; spontaneous oscillations.
Copyright © 2023 Li, He, Zhang, Li, Zhu, Jiao and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Box J. F. (1987). Guinness, gosset, fisher, and small samples. Stat. Sci. 2 45–52. 10.1214/ss/1177013437 - DOI
LinkOut - more resources
Full Text Sources

