Identification of TMEM53 as a novel SADS-CoV restriction factor that targets viral RNA-dependent RNA polymerase
- PMID: 37584551
- PMCID: PMC10467534
- DOI: 10.1080/22221751.2023.2249120
Identification of TMEM53 as a novel SADS-CoV restriction factor that targets viral RNA-dependent RNA polymerase
Abstract
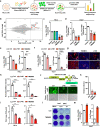
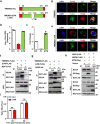
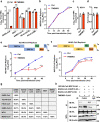
ABSTRACTZoonotic transmission of coronaviruses (CoVs) poses a serious public health threat. Swine acute diarrhea syndrome coronavirus (SADS-CoV), originating from a bat HKU2-related CoV, causes devastating swine diseases and poses a high risk of spillover to humans. Currently, licensed therapeutics that can prevent potential human outbreaks are unavailable. Identifying the cellular proteins that restrict viral infection is imperative for developing effective interventions and therapeutics. We utilized a large-scale human cDNA screening and identified transmembrane protein 53 (TMEM53) as a novel cell-intrinsic SADS-CoV restriction factor. The inhibitory effect of TMEM53 on SADS-CoV infection was found to be independent of canonical type I interferon responses. Instead, TMEM53 interacts with non-structural protein 12 (NSP12) and disrupts viral RNA-dependent RNA polymerase (RdRp) complex assembly by interrupting NSP8-NSP12 interaction, thus suppressing viral RdRp activity and RNA synthesis. Deleting the transmembrane domain of TMEM53 resulted in the abrogation of TMEM53-NSP12 interaction and TMEM53 antiviral activity. Importantly, TMEM53 exhibited broad antiviral activity against multiple HKU2-related CoVs. Our findings reveal a novel role of TMEM53 in SADS-CoV restriction and pave the way to host-directed therapeutics against HKU2-related CoV infection.
Keywords: HKU2-related CoVs; RdRp activity; SADS-CoV; TMEM53; coronavirus zoonosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
