Population Pharmacokinetic Analysis Supports Initiation Treatment and Bridging from Sublingual Buprenorphine to Subcutaneous Administration of a Buprenorphine Depot (CAM2038) in the Treatment of Opioid Use Disorder
- PMID: 37584841
- PMCID: PMC10520114
- DOI: 10.1007/s40262-023-01288-6
Population Pharmacokinetic Analysis Supports Initiation Treatment and Bridging from Sublingual Buprenorphine to Subcutaneous Administration of a Buprenorphine Depot (CAM2038) in the Treatment of Opioid Use Disorder
Abstract
Background and objective: In treating opioid use disorder (OUD), subcutaneous (SC) extended-release buprenorphine (BPN) depots, e.g., CAM2038, have been shown to provide smaller and less frequent fluctuations in BPN plasma concentrations and pharmacodynamic responses, improve outcomes, reduce treatment burden, and lower risks of misuse and diversion compared to daily sublingual (SL) BPN. This analysis characterized the pharmacokinetics (PK) of BPN following intravenous and SL administration, and administration of SC CAM2038 weekly and monthly.
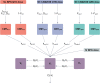
Methods: Pharmacokinetic data from two Phase 1 and two Phase 2 trials in healthy participants and participants with OUD, respectively, were used to develop a population PK model using non-linear mixed effects modelling. The analysis included data from 252 participants and 10,658 BPN observations.
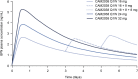
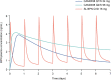
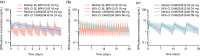
Results: The disposition of BPN was best described by a three-compartment model with first-order elimination, and absorption of SL BPN and SC CAM2038 weekly and monthly by dual parallel absorption pathways. Model diagnostics indicated good predictive performance of BPN concentrations. Buprenorphine plasma concentration-time profiles were simulated for treatment initiation, switching from SL BPN to CAM2038 weekly and monthly, and tapering after interrupting treatment with CAM2038. Simulations predicted CAM2038 weekly and monthly doses that provided BPN plasma maximum concentration (Cmax) and trough concentration (Ctrough) values at steady state within those observed following SL BPN administration.
Conclusions: This population PK model supports the use of CAM2038 doses as individualized treatment for OUD across different treatment stages, including initiation, switching from SL BPN according to established dose conversion schedules, and tapering.
Trial registrations: ISRCTN41550730 (05/19/2014), ISRCTN24987553 (07/29/2014), NCT02611752 (11/23/2015), NCT02710526 (03/16/2016).
© 2023. The Author(s).
Conflict of interest statement
MB: Current employee and stock owner of Pharmetheus AB. CA: Current employee of Pharmetheus AB. KS: Employee of Camurus AB at the time of the analysis. Currently a consultant in Pharmacokinetics and Pharmacometrics at PKadvisor AB. FT: Current employee and shareholder of Camurus AB.
Figures


References
-
- Dydyk AM, Jain NK, Gupta M. Opioid use disorder. Statpearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
-
- American Psychiatric Association . Substance-related and addictive disorders. Diagnostic and statistical manual of mental disorders: Dsm-5-tr. 5th Edition, Text. Revision. Washington, DC: American Psychiatric Association; 2022. pp. 543–665.
-
- Lintzeris N, Dunlop AJ, Haber PS, Lubman DI, Graham R, Hutchinson S, Arunogiri S, Hayes V, Hjelmström P, Svedberg A, Peterson S, Tiberg F. Patient-reported outcomes of treatment of opioid dependence with weekly and monthly subcutaneous depot vs daily sublingual buprenorphine: a randomized clinical trial. JAMA Netw. 2021;4(5):e219041. doi: 10.1001/jamanetworkopen.2021.9041. - DOI - PMC - PubMed
-
- Jones AK, Ngaimisi E, Gopalakrishnan M, Young MA, Laffont CM. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of Phase ii and Phase iii trials. Clin Pharmacokinet. 2021;60(4):527–540. doi: 10.1007/s40262-020-00957-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

