CDK4/6 inhibitors versus weekly paclitaxel for treatment of ER+/HER2- advanced breast cancer with impending or established visceral crisis
- PMID: 37584881
- PMCID: PMC10504109
- DOI: 10.1007/s10549-023-07035-6
CDK4/6 inhibitors versus weekly paclitaxel for treatment of ER+/HER2- advanced breast cancer with impending or established visceral crisis
Abstract
Purpose: ER+/HER2- advanced breast cancer (ABC) with visceral crisis (VC) or impending VC (IVC) is commonly treated with chemotherapy instead of CDK4/6 inhibitors (CDK4/6i). However, there is little evidence to confirm which treatment is superior. This study compared outcomes of patients with ER+/HER2- ABC and IVC/VC treated with CDK4/6i or weekly paclitaxel.
Methods: Patients with ER+/HER2- ABC receiving first line treatment at a large tertiary UK cancer centre from 1-Mar-2017 to 30-Jun-2021 were retrospectively identified. Hospital records were screened for IVC/VC affecting the liver, lungs/mediastinum, gastrointestinal tract and/or bone marrow. Baseline demographics, clinical data and survival outcomes were recorded up to 30-Jul-2022.
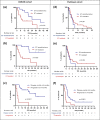
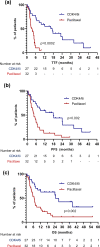
Results: 27/396 (6.8%) patients with ABC who received CDK4/6i and 32/86 (37.2%) who received paclitaxel had IVC/VC. Median time to treatment failure (TTF), progression-free survival (PFS) and overall survival (OS) were significantly longer in the CDK4/6i compared to paclitaxel cohort: TTF 17.3 vs. 3.5 months (HR 0.33, 95%CI 0.17-0.61, p = 0.0002), PFS 17.8 vs. 4.5 months (HR 0.38, 95%CI 0.21-0.67, p = 0.002), OS 24.6 vs. 6.7 months (HR 0.37, 95%CI 0.20-0.68, p = 0.002). The median time to first improvement in IVC/VC was similar in patients receiving CDK4/6i compared to paclitaxel (3.9 vs. 3.6 weeks, p = 0.773). Disease control at 4 months was not significantly different in the CDK4/6i and paclitaxel cohorts (77.8% vs. 59.4%, p = 0.168). In multivariate analysis, treatment with CDK4/6i was independently associated with a longer PFS compared to paclitaxel (HR 0.31, 95%CI 0.12-0.78, p = 0.015).
Conclusion: In this retrospective study, patients with ER+/HER2- ABC and IVC/VC treated with CDK4/6i had a significantly better survival compared to those treated with weekly paclitaxel. Further prospective studies that minimise possible selection bias are recommended.
Keywords: Advanced breast cancer; CDK4/6 inhibitor; Chemotherapy; ER positive; Paclitaxel; Visceral crisis.
© 2023. The Author(s).
Conflict of interest statement
RB: No competing interests to declare. ACA: Spousal shares in Astra Zeneca; Honoraria for advisory boards for MSD, Gilead and Astra Zeneca; Conference fees and travel expenses from Novartis; Research funding from Astra Zeneca paid to institution. SJH: Honoraria for speaking engagements for Pfizer, Novartis and Lilly; Honoraria for advisory boards for Lilly; Joint working agreement with Lilly and Novartis.
Figures
References
-
- Finn RS, Rugo HS, Dieras VC, Harbeck N, Im S-A, Gelmon KA, Walshe JM, Martin M, Chavez Mac Gregor M, Bananis E, Gauthier ER, Lu DR, Kim S, Slamon DJ. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC) JCO. 2022;40:1003–1003. doi: 10.1200/JCO.2022.40.17_suppl.LBA1003. - DOI
-
- Goetz MP, Toi M, Huober J, Sohn J, Tredan O, Park IH, Campone M, Chen SC, Sanchez LMM, Paluch-Shimon S, van Hal G, Shahir A, Iwata H, Johnston S. LBA15 MONARCH 3: Interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2-advanced breast cancer (ABC) Ann Oncol. 2022;33:S1384. doi: 10.1016/j.annonc.2022.08.009. - DOI - PubMed
-
- Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Hart L, Campone M, Petrakova K, Winer EP, Janni W, Conte P, Cameron DA, André F, Arteaga CL, Zarate JP, Chakravartty A, Taran T, Le Gac F, Serra P, O’Shaughnessy J. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386:942–950. doi: 10.1056/NEJMoa2114663. - DOI - PubMed
-
- Turner NC, Finn RS, Martin M, Im S-A, DeMichele A, Ettl J, Diéras V, Moulder S, Lipatov O, Colleoni M, Cristofanilli M, Lu DR, Mori A, Giorgetti C, Iyer S, Bartlett CH, Gelmon KA. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol. 2018;29:669–680. doi: 10.1093/annonc/mdx797. - DOI - PMC - PubMed
-
- Robertson JFR, Di Leo A, Johnston S, Chia S, Bliss JM, Paridaens RJ, Lichfield J, Bradbury I, Campbell C. Meta-analyses of visceral versus non-visceral metastatic hormone receptor-positive breast cancer treated by endocrine monotherapies. NPJ Breast Cancer. 2021;7:11. doi: 10.1038/s41523-021-00222-y. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

