Comparative Biophysical Study of Meibomian Lipids of Wild Type and Soat1-Null Mice: Implications to Meibomian Gland Dysfunction and Dry Eye Disease
- PMID: 37585190
- PMCID: PMC10434715
- DOI: 10.1167/iovs.64.11.20
Comparative Biophysical Study of Meibomian Lipids of Wild Type and Soat1-Null Mice: Implications to Meibomian Gland Dysfunction and Dry Eye Disease
Abstract
Purpose: The biophysical roles of Meibomian lipids (MLs) played in health and meibomian gland dysfunction (MGD) are still unclear. The purpose of this research is to establish the composition-structure-functional correlations of the ML film (MLF) using Soat1-null mice and comprehensive in vitro biophysical simulations.
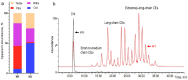
Methods: MLs were extracted from tarsal plates of wild type (WT) and Soat1 knockout (KO) mice. The chemical composition of ML samples was characterized using liquid chromatography - mass spectrometry. Comprehensive biophysical studies of the MLFs, including their dynamic surface activity, interfacial rheology, evaporation resistance, and ultrastructure and topography, were performed with a novel experimental methodology called the constrained drop surfactometry.
Results: Soat1 inactivation caused multiple alternations in the ML profile. Compared to their WT siblings, the MLs of KO mice were completely devoid of cholesteryl esters (CEs) longer than C18 to C20, but contained 7 times more free cholesterol (Chl). Biophysical assays consistently suggested that the KO-MLF became stiffer than that of WT mice, revealed by reduced film compressibility, increased elastic modulus, and decreased loss tangent, thus causing more energy loss per blinking cycle of the MLF. Moreover, the KO mice showed thinning of their MLF, and reduced evaporation resistance.
Conclusions: These findings delineated the composition-structure-functional correlations of the MLF and suggested a potential biophysical function of long-chain CEs in optimizing the surface activity, interfacial rheology, and evaporation resistance of the MLF. This study may provide novel implications to pathophysiological and translational understanding of MGD and dry eye disease.
Conflict of interest statement
Disclosure:
Figures





References
-
- King-Smith PE, Fink BA, Hill RM, Koelling KW, Tiffany JM.. The thickness of the tear film. Curr Eye Res. 2004; 29: 357–368. - PubMed
-
- Butovich I. Biochemistry and biophysics of human and animal tear film lipid layer: from composition to structure to function. Invest Ophthalmol Vis Sci. 2010; 51: 4154–4154.
-
- Georgiev GA, Eftimov P, Yokoi N.. Structure-function relationship of tear film lipid layer: a contemporary perspective. Exp Eye Res. 2017; 163: 17–28. - PubMed
-
- Cwiklik L. Tear film lipid layer: a molecular level view. Biochim Biophys Acta. 2016; 1858: 2421–2430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

