Perspectives on recent advancements in energy harvesting, sensing and bio-medical applications of piezoelectric gels
- PMID: 37585216
- PMCID: PMC10464879
- DOI: 10.1039/d3cs00202k
Perspectives on recent advancements in energy harvesting, sensing and bio-medical applications of piezoelectric gels
Abstract
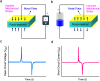
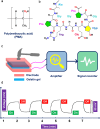
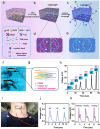
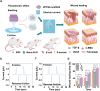
The development of next-generation bioelectronics, as well as the powering of consumer and medical devices, require power sources that are soft, flexible, extensible, and even biocompatible. Traditional energy storage devices (typically, batteries and supercapacitors) are rigid, unrecyclable, offer short-lifetime, contain hazardous chemicals and possess poor biocompatibility, hindering their utilization in wearable electronics. Therefore, there is a genuine unmet need for a new generation of innovative energy-harvesting materials that are soft, flexible, bio-compatible, and bio-degradable. Piezoelectric gels or PiezoGels are a smart crystalline form of gels with polar ordered structures that belongs to the broader family of piezoelectric material, which generate electricity in response to mechanical stress or deformation. Given that PiezoGels are structurally similar to hydrogels, they offer several advantages including intrinsic chirality, crystallinity, degree of ordered structures, mechanical flexibility, biocompatibility, and biodegradability, emphasizing their potential applications ranging from power generation to bio-medical applications. Herein, we describe recent examples of new functional PiezoGel materials employed for energy harvesting, sensing, and wound dressing applications. First, this review focuses on the principles of piezoelectric generators (PEGs) and the advantages of using hydrogels as PiezoGels in energy and biomedical applications. Next, we provide a detailed discussion on the preparation, functionalization, and fabrication of PiezoGel-PEGs (P-PEGs) for the applications of energy harvesting, sensing and wound healing/dressing. Finally, this review concludes with a discussion of the current challenges and future directions of P-PEGs.
Conflict of interest statement
There are no conflicts to declare.
Figures








Similar articles
-
Stretchable Polymer Hydrogels Based Flexible Triboelectric Nanogenerators for Self-Powered Bioelectronics.Biomacromolecules. 2025 Feb 10;26(2):787-813. doi: 10.1021/acs.biomac.4c01709. Epub 2025 Jan 8. Biomacromolecules. 2025. PMID: 39777943 Review.
-
Advancements in flexible biomechanical energy harvesting for smart health applications.Chem Commun (Camb). 2025 Feb 4;61(12):2424-2449. doi: 10.1039/d4cc05917d. Chem Commun (Camb). 2025. PMID: 39744849 Review.
-
Recent Progress on Hydrogel-Based Piezoelectric Devices for Biomedical Applications.Micromachines (Basel). 2023 Jan 9;14(1):167. doi: 10.3390/mi14010167. Micromachines (Basel). 2023. PMID: 36677228 Free PMC article. Review.
-
On-Body Piezoelectric Energy Harvesters through Innovative Designs and Conformable Structures.ACS Biomater Sci Eng. 2023 May 8;9(5):2070-2086. doi: 10.1021/acsbiomaterials.1c00800. Epub 2021 Nov 4. ACS Biomater Sci Eng. 2023. PMID: 34735770 Review.
-
Harvesting Inertial Energy and Powering Wearable Devices: A Review.Small Methods. 2024 Jan;8(1):e2300771. doi: 10.1002/smtd.202300771. Epub 2023 Oct 18. Small Methods. 2024. PMID: 37853661 Review.
Cited by
-
New Perspectives of Hydrogels in Chronic Wound Management.Molecules. 2025 Feb 4;30(3):686. doi: 10.3390/molecules30030686. Molecules. 2025. PMID: 39942790 Free PMC article. Review.
-
Stretchable, Self-Healing, and Bioactive Hydrogel with High-Functionality N,N'-bis(acryloyl)cystamine Dynamically Bonded Ag@polydopamine Crosslinkers for Wearable Sensors.Adv Sci (Weinh). 2024 Sep;11(35):e2404451. doi: 10.1002/advs.202404451. Epub 2024 Jul 19. Adv Sci (Weinh). 2024. PMID: 39031305 Free PMC article.
-
Advanced Materials for Energy Harvesting and Soft Robotics: Emerging Frontiers to Enhance Piezoelectric Performance and Functionality.Adv Mater. 2024 Nov;36(45):e2405363. doi: 10.1002/adma.202405363. Epub 2024 Sep 18. Adv Mater. 2024. PMID: 39291876 Review.
-
Piezoelectric Nanomaterials for Cancer Therapy: Current Research and Future Perspectives on Glioblastoma.J Funct Biomater. 2025 Mar 24;16(4):114. doi: 10.3390/jfb16040114. J Funct Biomater. 2025. PMID: 40278222 Free PMC article. Review.
-
Flexible mechano-optical dual-responsive perovskite molecular ferroelectric composites for advanced anticounterfeiting and encryption.Sci Adv. 2024 Nov 29;10(48):eadr2886. doi: 10.1126/sciadv.adr2886. Epub 2024 Nov 29. Sci Adv. 2024. PMID: 39612340 Free PMC article.
References
-
- Kim H. Pyun K. R. Lee M. T. Lee H. B. Ko S. H. Adv. Funct. Mater. 2022;32:2110535. doi: 10.1002/adfm.202110535. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

