Indolo[2,3- b]quinoxaline as a Low Reduction Potential and High Stability Anolyte Scaffold for Nonaqueous Redox Flow Batteries
- PMID: 37585274
- PMCID: PMC10472437
- DOI: 10.1021/jacs.3c05210
Indolo[2,3- b]quinoxaline as a Low Reduction Potential and High Stability Anolyte Scaffold for Nonaqueous Redox Flow Batteries
Abstract
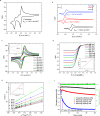
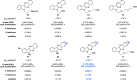
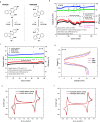
Redox flow batteries (RFBs) are a promising stationary energy storage technology for leveling power supply from intermittent renewable energy sources with demand. A central objective for the development of practical, scalable RFBs is to identify affordable and high-performance redox-active molecules as storage materials. Herein, we report the design, synthesis, and evaluation of a new organic scaffold, indolo[2,3-b]quinoxaline, for highly stable, low-reduction potential, and high-solubility anolytes for nonaqueous redox flow batteries (NARFBs). The mixture of 2- and 3-(tert-butyl)-6-(2-methoxyethyl)-6H-indolo[2,3-b]quinoxaline exhibits a low reduction potential (-2.01 V vs Fc/Fc+), high solubility (>2.7 M in acetonitrile), and remarkable stability (99.86% capacity retention over 49.5 h (202 cycles) of H-cell cycling). This anolyte was paired with N-(2-(2-methoxyethoxy)-ethyl)phenothiazine (MEEPT) to achieve a 2.3 V all-organic NARFB exhibiting 95.8% capacity retention over 75.1 h (120 cycles) of cycling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Masson-Delmotte V.; Zhai P.; Pörtner H. O.; Roberts D.; Skea J.; Shukla P. R.; Pirani A.; Moufouma-Okia W.; Péan C.; Pidcock R.; Connors S.. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, In the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty., 2018.
-
- Denholm P.; Ela E.; Kirby B.; Milligan M.. Role of Energy Storage with Renewable Electricity Generation; National Renewable Energy Laboratory: Golden, CO, USA, 2010.
-
- Johnson S. C.; Rhodes J. D.; Webber M. E. Understanding the Impact of Non-Synchronous Wind and Solar Generation on Grid Stability and Identifying Mitigation Pathways. Appl. Energy 2020, 262, 11449210.1016/j.apenergy.2020.114492. - DOI
-
- Liu W.; Lund H.; Mathiesen B. V. Large-Scale Integration of Wind Power into the Existing Chinese Energy System. Energy 2011, 36, 4753–4760. 10.1016/j.energy.2011.05.007. - DOI

