Endothelial FoxM1 reactivates aging-impaired endothelial regeneration for vascular repair and resolution of inflammatory lung injury
- PMID: 37585502
- PMCID: PMC10894510
- DOI: 10.1126/scitranslmed.abm5755
Endothelial FoxM1 reactivates aging-impaired endothelial regeneration for vascular repair and resolution of inflammatory lung injury
Abstract
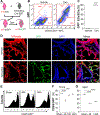
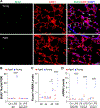
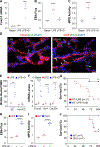
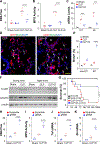
Aging is a major risk factor of high incidence and increased mortality of acute respiratory distress syndrome (ARDS). Here, we demonstrated that persistent lung injury and high mortality in aged mice after sepsis challenge were attributable to impaired endothelial regeneration and vascular repair. Genetic lineage tracing study showed that endothelial regeneration after sepsis-induced vascular injury was mediated by lung resident endothelial proliferation in young adult mice, whereas this intrinsic regenerative program was impaired in aged mice. Expression of forkhead box M1 (FoxM1), an important mediator of endothelial regeneration in young mice, was not induced in lungs of aged mice. Transgenic FOXM1 expression or in vivo endothelium-targeted nanoparticle delivery of the FOXM1 gene driven by an endothelial cell (EC)-specific promoter reactivated endothelial regeneration, normalized vascular repair and resolution of inflammation, and promoted survival in aged mice after sepsis challenge. In addition, treatment with the FDA-approved DNA demethylating agent decitabine was sufficient to reactivate FoxM1-dependent endothelial regeneration in aged mice, reverse aging-impaired resolution of inflammatory injury, and promote survival. Mechanistically, aging-induced Foxm1 promoter hypermethylation in mice, which could be inhibited by decitabine treatment, inhibited Foxm1 induction after sepsis challenge. In COVID-19 lung autopsy samples, FOXM1 was not induced in vascular ECs of elderly patients in their 80s, in contrast with middle-aged patients (aged 50 to 60 years). Thus, reactivation of FoxM1-mediated endothelial regeneration and vascular repair may represent a potential therapy for elderly patients with ARDS.
Conflict of interest statement
Figures




References
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, and Hudson LD, Incidence and outcomes of acute lung injury. N Engl J Med. 353, 1685–93 (2005). - PubMed
-
- Aird WC, The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 101, 3765–77 (2003). - PubMed
-
- Goldenberg NM, Steinberg BE, Slutsky AS, and Lee WL, Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med. 3, 88ps25 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

