Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination
- PMID: 37585505
- PMCID: PMC11137749
- DOI: 10.1126/scitranslmed.abq0603
Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination
Abstract
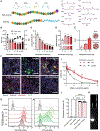
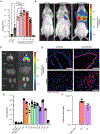
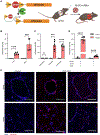
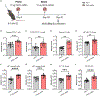
An inhalable platform for messenger RNA (mRNA) therapeutics would enable minimally invasive and lung-targeted delivery for a host of pulmonary diseases. Development of lung-targeted mRNA therapeutics has been limited by poor transfection efficiency and risk of vehicle-induced pathology. Here, we report an inhalable polymer-based vehicle for delivery of therapeutic mRNAs to the lung. We optimized biodegradable poly(amine-co-ester) (PACE) polyplexes for mRNA delivery using end-group modifications and polyethylene glycol. These polyplexes achieved high transfection of mRNA throughout the lung, particularly in epithelial and antigen-presenting cells. We applied this technology to develop a mucosal vaccine for severe acute respiratory syndrome coronavirus 2 and found that intranasal vaccination with spike protein-encoding mRNA polyplexes induced potent cellular and humoral adaptive immunity and protected susceptible mice from lethal viral challenge. Together, these results demonstrate the translational potential of PACE polyplexes for therapeutic delivery of mRNA to the lungs.
Conflict of interest statement
Figures
Update of
-
Inhalable polymer nanoparticles for versatile mRNA delivery and mucosal vaccination.bioRxiv [Preprint]. 2022 Mar 23:2022.03.22.485401. doi: 10.1101/2022.03.22.485401. bioRxiv. 2022. Update in: Sci Transl Med. 2023 Aug 16;15(709):eabq0603. doi: 10.1126/scitranslmed.abq0603. PMID: 35350207 Free PMC article. Updated. Preprint.
Comment in
-
Delivering mRNA to the lungs.Nat Rev Drug Discov. 2023 Oct;22(10):787. doi: 10.1038/d41573-023-00142-5. Nat Rev Drug Discov. 2023. PMID: 37666973 No abstract available.
References
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr., Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). - PMC - PubMed
-
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

