Luteal Lipid Droplets: A Novel Platform for Steroid Synthesis
- PMID: 37586092
- PMCID: PMC10445418
- DOI: 10.1210/endocr/bqad124
Luteal Lipid Droplets: A Novel Platform for Steroid Synthesis
Abstract
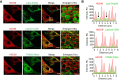
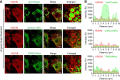
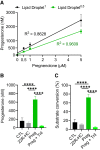
Progesterone is an essential steroid hormone that is required to initiate and maintain pregnancy in mammals and serves as a metabolic intermediate in the synthesis of endogenously produced steroids, including sex hormones and corticosteroids. Steroidogenic luteal cells of the corpus luteum have the tremendous capacity to synthesize progesterone. These specialized cells are highly enriched with lipid droplets that store lipid substrate, which can be used for the synthesis of steroids. We recently reported that hormone-stimulated progesterone synthesis by luteal cells requires protein kinase A-dependent mobilization of cholesterol substrate from lipid droplets to mitochondria. We hypothesize that luteal lipid droplets are enriched with steroidogenic enzymes and facilitate the synthesis of steroids in the corpus luteum. In the present study, we analyzed the lipid droplet proteome, conducted the first proteomic analysis of lipid droplets under acute cyclic adenosine monophosphate (cAMP)-stimulated conditions, and determined how specific lipid droplet proteins affect steroidogenesis. Steroidogenic enzymes, cytochrome P450 family 11 subfamily A member 1 and 3 beta-hydroxysteroid dehydrogenase (HSD3B), were highly abundant on lipid droplets of the bovine corpus luteum. High-resolution confocal microscopy confirmed the presence of active HSD3B on the surface of luteal lipid droplets. We report that luteal lipid droplets have the capacity to synthesize progesterone from pregnenolone. Lastly, we analyzed the lipid droplet proteome following acute stimulation with cAMP analog, 8-Br-cAMP, and report increased association of HSD3B with luteal lipid droplets following stimulation. These findings provide novel insights into the role of luteal lipid droplets in steroid synthesis.
Keywords: bovine; corpus luteum; lipid droplets; mitochondria; progesterone; protein kinase A; steroidogenesis.
Published by Oxford University Press on behalf of the Endocrine Society 2023.
Figures



References
-
- Quinkler M, Meyer B, Bumke-Vogt C, et al. . Agonistic and antagonistic properties of progesterone metabolites at the human mineralocorticoid receptor. Eur J Endocrinol. 2002;146(6):789‐799. - PubMed
-
- Takao Y, Honda T, Ueda M, et al. . Immunohistochemical localization of the LH/HCG receptor in human ovary: HCG enhances cell surface expression of LH/HCG receptor on luteinizing granulosa cells in vitro. Mol Hum Reprod. 1997;3(7):569‐578. - PubMed
-
- Marsh JM. The role of cyclic AMP in gonadal steroidogenesis. Biol Reprod. 1976;14(1):30‐53. - PubMed

