Phase 1 clinical trial to assess safety and efficacy of NY-ESO-1-specific TCR T cells in HLA-A∗02:01 patients with advanced soft tissue sarcoma
- PMID: 37586317
- PMCID: PMC10439245
- DOI: 10.1016/j.xcrm.2023.101133
Phase 1 clinical trial to assess safety and efficacy of NY-ESO-1-specific TCR T cells in HLA-A∗02:01 patients with advanced soft tissue sarcoma
Abstract
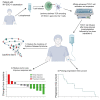
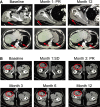
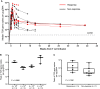
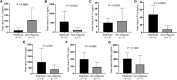
New York esophageal squamous cell carcinoma-1 (NY-ESO-1)-specific T cell receptor (TCR) T cell therapy is effective in tumors with NY-ESO-1 expression, but a safe and effective TCR-T cell therapeutic protocol remains to be improved. Here, we report a phase 1 investigational new drug clinical trial with TCR affinity-enhanced specific T cell therapy (TAEST16001) for targeting NY-ESO-1. Enrolled patients receive TAEST16001 cell infusion after dose-reduced lymphodepletion with cyclophosphamide (15 mg/kg/day × 3 days) combined with fludarabine (20 mg/m2/day × 3 days), and the TCR-T cells are maintained with low doses of interleukin-2 injection post-adoptive transfer. Analysis of 12 patients treated with the regimen demonstrates no treatment-related serious adverse events. The overall response rate is 41.7%. The median progression-free survival is 7.2 months, and the median duration of response is 13.1 months. The protocol of TAEST16001 cells delivers a safe and highly effective treatment for patients with advanced soft tissue sarcoma (ClinicalTrials.gov: NCT04318964).
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Z.H., Y.O., L.Z., H.G., A.C., J.C., S.Z., Y. Lin, Y. Li, and K.M. are employees of Xiangxue Life Science Technology (Guangdong) Co., Ltd.
Figures


References
-
- von Mehren M., Randall R.L., Benjamin R.S., Boles S., Bui M.M., Ganjoo K.N., George S., Gonzalez R.J., Heslin M.J., Kane J.M., et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018;16:536–563. doi: 10.6004/jnccn.2018.0025. - DOI - PubMed
-
- Sleijfer S., Ouali M., van Glabbeke M., Krarup-Hansen A., Rodenhuis S., Le Cesne A., Hogendoorn P.C.W., Verweij J., Blay J.Y. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) Eur. J. Cancer. 2010;46:72–83. doi: 10.1016/j.ejca.2009.09.022. - DOI - PubMed
-
- Gronchi A., Miah A.B., Dei Tos A.P., Abecassis N., Bajpai J., Bauer S., Biagini R., Bielack S., Blay J.Y., Bolle S., et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021;32:1348–1365. doi: 10.1016/j.annonc.2021.07.006. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

