Map-based B0 shimming for single voxel brain spectroscopy at 7T
- PMID: 37586403
- PMCID: PMC12056978
- DOI: 10.1002/nbm.5021
Map-based B0 shimming for single voxel brain spectroscopy at 7T
Abstract
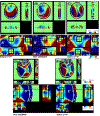
While B0 shimming is an important requirement for in vivo brain spectroscopy, for single voxel spectroscopy (SVS), the role for advanced shim methods has been questioned. Specifically, with the small spatial dimensions of the voxel, the extent to which inhomogeneities higher than second order exist and the ability of higher order shims to correct them is controversial. To assess this, we acquired SVS from two loci of neurophysiological interest, the rostral prefrontal cortex (rPFC; 8 cc) and hippocampus (Hc; 9 cc). The rPFC voxel was placed using SUsceptibility Managed Optimization (SUMO) and an initial B0 map that covers the entire cerebrum to cerebellum. In each location, we compared map-based shimming (Bolero) with projection-based shimming (FAST(EST)MAP). We also compared vendor-provided spherical harmonic first- and second-order shims with additional third- and fourth-order shim hardware. The 7T SVS acquisition used stimulated echo acquisition mode (STEAM) TR/TM/TE of 6 s/20 ms/8 ms, a tissue water acquisition for concentration reference, and LCModel for spectral analysis. In the rPFC (n = 7 subjects), Bolero shimming with first- and second-order shims reduced the residual inhomogeneity from 9.8 ± 4.5 Hz with FAST(EST)MAP to 6.5 ± 2.0 Hz. The addition of third- and fourth-order shims further reduced to 4.0 ± 0.8 Hz. In the Hc (n = 7 subjects), FAST(EST)MAP, Bolero with first- and second-order shims, and Bolero with first- to fourth-order shims achieved values of 8.6 ± 1.9, 5.6 ± 1.0, and 4.6 ± 0.9 Hz, respectively. The spectral linewidth, , was estimated with a Voigt lineshape using and T2 = 130 ms. significantly correlated with the Cramer-Rao lower bounds and concentrations of several metabolites, including glutamate and glutamine in the rPFC. In both loci, if the B0 distribution is well described by a Gaussian model, the variance of the metabolite concentrations is reduced, consistent with the LCModel fit based on a unimodal lineshape. Overall, the use of the high order and map-based B0 shim methods improved the accuracy and consistency of spectroscopic data.
Keywords: 7T; CRLB; linewidth; shimming; spectroscopy; spherical harmonic.
© 2023 The Authors. NMR in Biomedicine published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
H. P. Hetherington PhD is an employee of Resonance Research Inc., who is the vendor of the high order shim coil and RF coil. H. P. Hetherington is also Adjunct Professor of Radiology at University of Missouri Columbia.
Figures



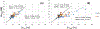


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

