SARS-CoV-2 seroprevalence in pregnant women during the first three COVID-19 waves in The Gambia
- PMID: 37586660
- PMCID: PMC7618177
- DOI: 10.1016/j.ijid.2023.08.012
SARS-CoV-2 seroprevalence in pregnant women during the first three COVID-19 waves in The Gambia
Abstract
Objectives: SARS-CoV-2 transmission in sub-Saharan Africa has probably been underestimated. Population-based seroprevalence studies are needed to determine the extent of transmission in the continent.
Methods: Blood samples from a cohort of Gambian pregnant women were tested for SARS-CoV-2 total receptor binding domain (RBD) immunoglobulin (Ig) M/IgG before (Pre-pandemic: October-December 2019) and during the pandemic (Pre-wave 1: February-June 2020; Post-wave 1: October-December 2020, Post-wave 2: May-June 2021; and Post-wave 3: October-December 2021). Samples reactive for SARS-CoV-2 total RBD IgM/IgG were tested in specific S1- and nucleocapsid (NCP) IgG assays.
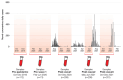
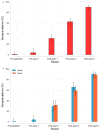
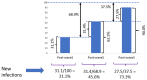
Results: SARS-CoV-2 total RBD IgM/IgG seroprevalence was 0.9% 95% confidence interval (0.2, 4.9) in Pre-pandemic; 4.1% (1.4, 11.4) in Pre-wave 1; 31.1% (25.2, 37.7) in Post-wave 1; 62.5% (55.8, 68.8) in Post-wave 2 and 90.0% (85.1, 93.5) in Post-wave 3. S-protein IgG and NCP-protein IgG seroprevalence also increased at each Post-wave period. Although S-protein IgG and NCP-protein IgG seroprevalence was similar at Post-wave 1, S-protein IgG seroprevalence was higher at Post-wave 2 and Post-wave 3, (prevalence difference 13.5 [0.1, 26.8] and prevalence ratio 1.5 [1.0, 2.3] in Post-wave 2; and 22.9 [9.2, 36.6] and 1.4 [1.1, 1.8] in Post-wave 3 respectively, P <0.001).
Conclusion: SARS-CoV-2 transmission in The Gambia during the first 3 COVID-19 waves was high, differing significantly from official numbers of COVID-19 cases reported. Our findings are important for policy makers in managing the near-endemic COVID-19.
Keywords: Antibodies; COVID-19; Pregnancy; Seroprevalence; The Gambia; sub-Saharan Africa.
Copyright © 2023. Published by Elsevier Ltd.
Conflict of interest statement
Declarations of Competing Interest The authors have no competing interests to declare.
Figures

References
-
- World Health Organization. COVID-19 weekly epidemiological update. 72nd ed. 2021. [accessed 08 August 2022]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on... .
-
- World Health Organization, director. General’s opening remarks at the media briefing on COVID-19. 2020. [accessed 30 June 2021]. https://www.who.int/director-general/speeches/detail/who-director-genera... .
-
- Adepoju P. Nigeria responds to COVID-19; first case detected in sub-Saharan Africa. Nat Med. 2020;26:444–8. - PubMed
-
- Frost I, Osena G, Craig J, Hauck S, Kalanxhi E, Gatalo O, et al. COVID-19 in West Africa: National Projections of Total and Severe Infections Under Different Lockdown Scenarios. 2020. [accessed 06 July 2021]. https://cddep.org/wp-content/uploads/2020/07/West-Africa.pdf . - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

