PPARγ activation modulates the balance of peritoneal macrophage populations to suppress ovarian tumor growth and tumor-induced immunosuppression
- PMID: 37586764
- PMCID: PMC10432661
- DOI: 10.1136/jitc-2023-007031
PPARγ activation modulates the balance of peritoneal macrophage populations to suppress ovarian tumor growth and tumor-induced immunosuppression
Abstract
Background: Ovarian adenocarcinoma (OVAD) frequently metastasizes to the peritoneal cavity and manifests by the formation of ascites, which constitutes a tumor-promoting microenvironment. In the peritoneal cavity, two developmentally, phenotypically and functionally distinct macrophage subsets, immunocompetent large peritoneal macrophages (LPM) and immunosuppressive small peritoneal macrophages (SPM), coexist. Because peroxisome proliferator-activated receptor γ (PPARγ) is a critical factor participating in macrophage differentiation and cooperates with CCAAT/enhancer binding protein β (C/EBPβ), a transcription factor essential for SPM-to-LPM differentiation, PPARγ could be also involved in the regulation of SPM/LPM balance and could be a promising therapeutic target.
Methods: To evaluate the 15(S)-hydroxyeicosatetraenoic acid (HETE), a PPARγ endogenous ligand, impact on ovarian tumor growth, we intraperitoneally injected 15(S)-HETE into a murine ovarian cancer model. This experimental model consists in the intraperitoneally injection of ID8 cells expressing luciferase into syngeneic C57BL/6 female mice. This ID8 orthotopic mouse model is a well-established experimental model of end-stage epithelial OVAD. Tumor progression was monitored using an in vivo imaging system. Peritoneal immune cells in ascites were analyzed by flow cytometry and cell sorting. To determine whether the impact of 15(S)-HETE in tumor development is mediated through the macrophages, these cells were depleted by injection of liposomal clodronate. To further dissect how 15(S)-HETE mediated its antitumor effect, we assessed the tumor burden in tumor-bearing mice in which the PPARγ gene was selectively disrupted in myeloid-derived cells and in mice deficient of the recombination-activating gene Rag2. Finally, to validate our data in humans, we isolated and treated macrophages from ascites of individuals with OVAD.
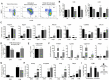
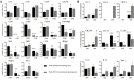
Results: Here we show, in the murine experimental model of OVAD, that 15(S)-HETE treatment significantly suppresses the tumor growth, which is associated with the differentiation of SPM into LPM and the LPM residency in the peritoneal cavity. We demonstrate that C/EBPβ and GATA6 play a central role in SPM-to-LPM differentiation and in LPM peritoneal residence through PPARγ activation during OVAD. Moreover, this SPM-to-LPM switch is associated with the increase of the effector/regulatory T-cell ratio. Finally, we report that 15(S)-HETE attenuates immunosuppressive properties of human ovarian tumor-associated macrophages from ascites.
Conclusion: Altogether, these results promote PPARγ as a potential therapeutic target to restrain OVAD development and strengthen the use of PPARγ agonists in anticancer therapy.
Keywords: Immunity, Innate; Inflammation; Macrophages; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures






References
-
- Risch HA, Howe GR. Pelvic inflammatory disease and the risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 1995;4:447–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
