ScRNA-seq defines dynamic T-cell subsets in longitudinal colon and peripheral blood samples in immune checkpoint inhibitor-induced colitis
- PMID: 37586769
- PMCID: PMC10432652
- DOI: 10.1136/jitc-2023-007358
ScRNA-seq defines dynamic T-cell subsets in longitudinal colon and peripheral blood samples in immune checkpoint inhibitor-induced colitis
Abstract
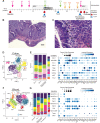
Immune checkpoint inhibitors (ICIs) are increasingly being used to manage multiple tumor types. Unfortunately, immune-related adverse events affect up to 60% of recipients, often leading to treatment discontinuation in settings where few alternative cancer therapies may be available. Checkpoint inhibitor induced colitis (ICI-colitis) is a common toxicity for which the underlying mechanisms are poorly defined. To better understand the changing colon-specific and peripheral immune environments over the course of progression and treatment of colitis, we collected blood and colon tissue from a patient with Merkel cell carcinoma who developed colitis on treatment with pembrolizumab. We performed single-cell RNA sequencing and T-cell receptor sequencing on samples collected before, during and after pembrolizumab and after various interventions to mitigate toxicity. We report T-cells populations defined by cytotoxicity, memory, and proliferation markers at various stages of colitis. We show preferential depletion of CD8+ T cells with biologic therapy and nominate both circulating and colon-resident T-cell subsets as potential drivers of inflammation and response to immune suppression. Our findings highlight the need for further exploration of the colon immune environment and rationalize future studies evaluating biologics for ICI-colitis, including in the context of ICI re-challenge.
Keywords: CD8-Positive T-Lymphocytes; Immune Checkpoint Inhibitors; Immunotherapy; Programmed Cell Death 1 Receptor; T-Lymphocytes.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MER reports consultant fees from Takeda, Bristol-Myers Squibb, and ImmunogenX. DH received research funding from Bristol-Myers Squibb, Sanofi, and Genentech. He has been a consultant for Bristol-Myers Squibb, Compass Therapeutics, EMD Serono, Genentech, Juno Therapeutics, Novartis Pharmaceuticals, Proclara Biosciences, Sage Therapeutics, and Sanofi Genzyme. HMK reports institutional research grants from Merck, Bristol-Myers Squibb and Apexigen and financial support from Iovance, Celldex, Merck, Elevate Bio, Instil Bio, Bristol-Myers Squibb, Clinigen, Shionogi, Chemocentryx, Calithera, Signatero, Gigagen, GI reviewers, Seranova, Pliant Therapeutics. No other disclosures were reported.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
