CLN-978, a novel half-life extended CD19/CD3/HSA-specific T cell-engaging antibody construct with potent activity against B-cell malignancies with low CD19 expression
- PMID: 37586770
- PMCID: PMC10432633
- DOI: 10.1136/jitc-2023-007398
CLN-978, a novel half-life extended CD19/CD3/HSA-specific T cell-engaging antibody construct with potent activity against B-cell malignancies with low CD19 expression
Erratum in
-
Correction: CLN-978, a novel half-life extended CD19/CD3/HSA-specific T cell-engaging antibody construct with potent activity against B-cell malignancies with low CD19 expression.J Immunother Cancer. 2023 Dec 14;11(12):e007398corr1. doi: 10.1136/jitc-2023-007398corr1. J Immunother Cancer. 2023. PMID: 38101863 Free PMC article. No abstract available.
Abstract
Background: Despite significant progress in the development of T cell-engaging therapies for various B-cell malignancies, a high medical need remains for the refractory disease setting, often characterized by suboptimal target levels.
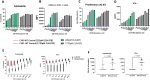
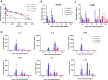
Methods: To address this issue, we have developed a 65-kDa multispecific antibody construct, CLN-978, with affinities tuned to optimize the killing of low-CD19 expressing tumor cells. CLN-978 bound to CD19 on B cells with picomolar affinity, and to CD3ε on T cells with nanomolar affinity. A serum albumin binding domain was incorporated to extend serum half-life. In this setting, we biophysically characterize and report the activities of CLN-978 in cell co-culture assays, multiple mouse models and non-human primates.
Results: Human T cells redirected by CLN-978 could eliminate target cells expressing less than 300 copies of CD19 on their surface. The half-life extension and high affinity for CD19 led to significant antitumor activity in murine lymphoma models at very low doses of CLN-978. In primates, we observed a long serum half-life, deep and sustained depletion of normal B cells, and remarkable tolerability, in particular, reduced cytokine release when CLN-978 was administered subcutaneously.
Conclusions: CLN-978 warrants further exploration. An ongoing clinical phase 1 trial is investigating safety, pharmacokinetics, pharmacodynamics, and the initial therapeutic potential of subcutaneously administered CLN-978 in patients with non-Hodgkin's lymphoma.
Keywords: CD4-Positive T-Lymphocytes; CD8-Positive T-Lymphocytes; Cytotoxicity, Immunologic; Drug Evaluation, Preclinical; T-Lymphocytes.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NKM, KM, BL, PAB, and JSM have ownership in Cullinan Oncology, which is seeking to commercialize CLN-978.
Figures




References
-
- Madduri D, Rosko A, Brayer J, et al. Regn5458, a BCMA X CD3 bispecific monoclonal antibody, induces deep and durable responses in patients with relapsed/refractory multiple myeloma (RRMM). Blood 2020;136:41–2. 10.1182/blood-2020-139192 10.1182/blood-2020-139192 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
