3-year follow-up of a prospective, multicenter study of the Amplatzer Piccolo™ Occluder for transcatheter patent ductus arteriosus closure in children ≥ 700 grams
- PMID: 37587183
- PMCID: PMC10541325
- DOI: 10.1038/s41372-023-01741-1
3-year follow-up of a prospective, multicenter study of the Amplatzer Piccolo™ Occluder for transcatheter patent ductus arteriosus closure in children ≥ 700 grams
Abstract
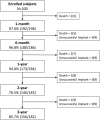
Objective: This study describes 3-year follow-up of 200 infants weighing ≥ 700 grams who underwent transcatheter patent ductus arteriosus (PDA) closure with the Amplatzer Piccolo™ Occluder.
Study design: Between June 2017 and February 2019, 200 children were enrolled in this U.S. study (NCT03055858). PDA closure, survival, and device- or procedure-related events were evaluated. A total of 156 of the available 182 patients (86%) completed the study.
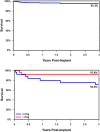
Results: The implant success rate was 95.5% (191/200). At 3 years, PDA closure was observed in 100% (33/33) of patients. Survival was >95% with 9 reported deaths. No deaths were adjudicated as device- or procedure-related. Notable events included aortic obstruction (2) requiring stent placement and tricuspid regurgitation (5), for which no interventions were required.
Conclusions: This follow-up study demonstrates high rates of PDA closure, low serious complication rates, and survival > 95% at 3 years. The Amplatzer Piccolo™ Occluder is a safe and effective therapy for PDA treatment in premature infants.
Clinicaltrials: gov identifier: NCT0305585.
© 2023. The Author(s).
Conflict of interest statement
BHM is a consultant and proctor for Abbott Structural Heart; SS: proctor/consultant Abbott; TF: proctor/consultant Abbott, Edwards, AcuNav/Biosence Webster, B. Braun Medical, Siemens, Medtronic; MG: proctor/consultant Abbott, Medtronic, W.L. Gore & Assoc; DB: proctor/consultant Abbott, Edwards, Medtronic; AKA: proctor/consultant Abbott, Edwards, Medtronic, Cook Medical; S. Shahanavaz: proctor Abbott, Medtronic, and Edwards; TJ: research grant, proctor/consultant Abbott, Edwards, Medtronic, W.L. Gore & Assoc.; BM: Consultant Medtronic, proctor Abbott; TR: proctor Abbott; HJ: proctor/consultant Abbott, Baylis Medtech, Chiesi USA, Edwards Lifesciences, Medtronic, Clinical trial executive committee Janssen Pharmaceutical, Co-founder PolyVascular, scientific advisory board Pediastent; DN: proctor Abbott, consultant and independent data reviewer W.L. Gore & Assoc, expert witness Glaxo Smith Kline; CW: full-time Abbott employee; DG: full-time employee Abbott; EMZ: consultant/proctor Abbott, Edwards, Medtronic, National PI ADO II AS IDE Trial and Alterra/S3.
Figures


References
-
- Benitz WE, Committee on Fetus and Newborn. Patent Ductus Arteriosus in Preterm Infants. Pediatrics. 2016;137:e20153730. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

