Platelet factors are induced by longevity factor klotho and enhance cognition in young and aging mice
- PMID: 37587231
- PMCID: PMC10501899
- DOI: 10.1038/s43587-023-00468-0
Platelet factors are induced by longevity factor klotho and enhance cognition in young and aging mice
Abstract
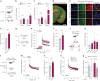
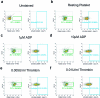
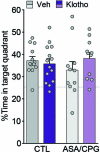
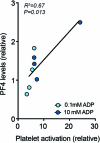
Platelet factors regulate wound healing and can signal from the blood to the brain1,2. However, whether platelet factors modulate cognition, a highly valued and central manifestation of brain function, is unknown. Here we show that systemic platelet factor 4 (PF4) permeates the brain and enhances cognition. We found that, in mice, peripheral administration of klotho, a longevity and cognition-enhancing protein3-7, increased the levels of multiple platelet factors in plasma, including PF4. A pharmacologic intervention that inhibits platelet activation blocked klotho-mediated cognitive enhancement, indicating that klotho may require platelets to enhance cognition. To directly test the effects of platelet factors on the brain, we treated mice with vehicle or systemic PF4. In young mice, PF4 enhanced synaptic plasticity and cognition. In old mice, PF4 decreased cognitive deficits and restored aging-induced increases of select factors associated with cognitive performance in the hippocampus. The effects of klotho on cognition were still present in mice lacking PF4, suggesting this platelet factor is sufficient to enhance cognition but not necessary for the effects of klotho-and that other unidentified factors probably contribute. Augmenting platelet factors, possible messengers of klotho, may enhance cognition in the young brain and decrease cognitive deficits in the aging brain.
© 2023. The Author(s).
Conflict of interest statement
The Regents of the University of California hold an issued patent on ‘Methods and compositions for improved cognition’ involving klotho (US10864256B2, Inventor D.B.D.) and have applied for a provisional patent applications related to the content of the manuscript findings, ‘Platelet factors and cognitive improvement’ (PCT/US2021/017580, coinventors C.P., S.V. and D.B.D.) and ‘Use of downstream factors in the klotho pathway to assess klotho activity’ (PCT/US2021/020706, inventor D.B.D.). The rest of the authors (O.H., S.G., A.J.M., F.M., B.K. and T.W.-C.) have no competing interests related to the manuscript. D.B.D. consulted for Unity Biotechnology (unrelated to content of manuscript) and SV Health Investors.
Figures









Comment in
-
Platelets rejuvenate the aging brain.Nat Cardiovasc Res. 2023 Oct;2(10):859. doi: 10.1038/s44161-023-00355-2. Nat Cardiovasc Res. 2023. PMID: 39196256 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

