Xiphoid nucleus of the midline thalamus controls cold-induced food seeking
- PMID: 37587337
- PMCID: PMC10482681
- DOI: 10.1038/s41586-023-06430-9
Xiphoid nucleus of the midline thalamus controls cold-induced food seeking
Abstract
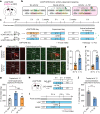
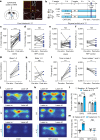
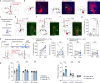
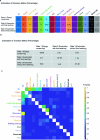
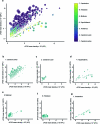
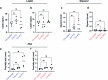
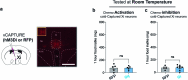
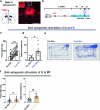
Maintaining body temperature is calorically expensive for endothermic animals1. Mammals eat more in the cold to compensate for energy expenditure2, but the neural mechanism underlying this coupling is not well understood. Through behavioural and metabolic analyses, we found that mice dynamically switch between energy-conservation and food-seeking states in the cold, the latter of which are primarily driven by energy expenditure rather than the sensation of cold. To identify the neural mechanisms underlying cold-induced food seeking, we used whole-brain c-Fos mapping and found that the xiphoid (Xi), a small nucleus in the midline thalamus, was selectively activated by prolonged cold associated with elevated energy expenditure but not with acute cold exposure. In vivo calcium imaging showed that Xi activity correlates with food-seeking episodes under cold conditions. Using activity-dependent viral strategies, we found that optogenetic and chemogenetic stimulation of cold-activated Xi neurons selectively recapitulated food seeking under cold conditions whereas their inhibition suppressed it. Mechanistically, Xi encodes a context-dependent valence switch that promotes food-seeking behaviours under cold but not warm conditions. Furthermore, these behaviours are mediated by a Xi-to-nucleus accumbens projection. Our results establish Xi as a key region in the control of cold-induced feeding, which is an important mechanism in the maintenance of energy homeostasis in endothermic animals.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Update of
-
Xiphoid nucleus of the midline thalamus controls cold-induced food seeking.bioRxiv [Preprint]. 2023 Mar 18:2023.03.16.533067. doi: 10.1101/2023.03.16.533067. bioRxiv. 2023. Update in: Nature. 2023 Sep;621(7977):138-145. doi: 10.1038/s41586-023-06430-9. PMID: 36993706 Free PMC article. Updated. Preprint.
References
-
- Blaxter, K. Energy Metabolism in Animals and Man (Cambridge Univ. Press, 1989).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

