Platelet factors attenuate inflammation and rescue cognition in ageing
- PMID: 37587343
- PMCID: PMC10468395
- DOI: 10.1038/s41586-023-06436-3
Platelet factors attenuate inflammation and rescue cognition in ageing
Abstract
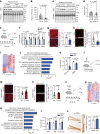
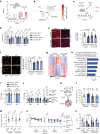
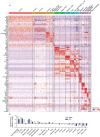
Identifying therapeutics to delay, and potentially reverse, age-related cognitive decline is critical in light of the increased incidence of dementia-related disorders forecasted in the growing older population1. Here we show that platelet factors transfer the benefits of young blood to the ageing brain. Systemic exposure of aged male mice to a fraction of blood plasma from young mice containing platelets decreased neuroinflammation in the hippocampus at the transcriptional and cellular level and ameliorated hippocampal-dependent cognitive impairments. Circulating levels of the platelet-derived chemokine platelet factor 4 (PF4) (also known as CXCL4) were elevated in blood plasma preparations of young mice and humans relative to older individuals. Systemic administration of exogenous PF4 attenuated age-related hippocampal neuroinflammation, elicited synaptic-plasticity-related molecular changes and improved cognition in aged mice. We implicate decreased levels of circulating pro-ageing immune factors and restoration of the ageing peripheral immune system in the beneficial effects of systemic PF4 on the aged brain. Mechanistically, we identified CXCR3 as a chemokine receptor that, in part, mediates the cellular, molecular and cognitive benefits of systemic PF4 on the aged brain. Together, our data identify platelet-derived factors as potential therapeutic targets to abate inflammation and rescue cognition in old age.
© 2023. The Author(s).
Conflict of interest statement
The regents of the University of California have applied for a provisional patent application arising from this work (‘Platelet factors and cognitive improvement’; PCT/US2021/017580; A.B.S., P.B.V. and S.A.V. are listed as coinventors). S.A.V. consulted for SV Health Investors and The Herrick Company. The other authors declare no competing interests.
Figures




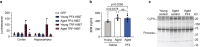




Comment in
-
"Bloody" good factors for keeping the brain young.Immunity. 2023 Oct 10;56(10):2185-2187. doi: 10.1016/j.immuni.2023.09.007. Immunity. 2023. PMID: 37820581
-
Platelets rejuvenate the aging brain.Nat Cardiovasc Res. 2023 Oct;2(10):859. doi: 10.1038/s44161-023-00355-2. Nat Cardiovasc Res. 2023. PMID: 39196256 No abstract available.
References
-
- James SL, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

