Multi-target regulatory mechanism of Yang Xin Tang - a traditional Chinese medicine against dementia
- PMID: 37587513
- PMCID: PMC10428601
- DOI: 10.1186/s13020-023-00813-w
Multi-target regulatory mechanism of Yang Xin Tang - a traditional Chinese medicine against dementia
Abstract
Background: Yang Xin Tang (YXT) is a traditional Chinese herbal preparation which has been reported to improve cognitive function and memory in patients with dementia. As the underlying mechanism of action of YXT has not been elucidated, we examined the effects of YXT and its major herbal components in regulating gene transcription and molecular targets related to Alzheimer's disease (AD).
Methods: Aqueous and ethanol extracts of YXT and selected herbal components were prepared and validated by standard methods. A series of biochemical and cellular assays were employed to assess the ability of the herbal extracts to inhibit acetylcholinesterase, reduce β-amyloid aggregation, stimulate the differentiation of neural progenitor cells, suppress cyclooxygenase, and protect neurons against β-amyloid or N-methyl-D-aspartate-induced cytotoxicity. The effects of YXT on multiple molecular targets were further corroborated by a panel of nine reporter gene assays.
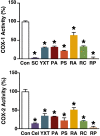
Results: Extracts of YXT and two of its constituent herbs, Poria cocos and Poria Sclerotium pararadicis, significantly inhibited β-amyloid aggregation and β-amyloid-induced cytotoxicity. A protective effect of the YXT extract was similarly observed against N-methyl-D-aspartate-induced cytotoxicity in primary neurons, and this activity was shared by extracts of Radix Astragali and Rhizoma Chuanxiong. Although the YXT extract was ineffective, extracts of Poria cocos, Poria Sclerotium pararadicis and Radix Polygalae inhibited acetylcholine esterase, with the latter also capable of upregulating choline acetyltransferase. YXT and its components significantly inhibited the activities of the pro-inflammatory cyclooxygenases. Additionally, extracts of YXT and several of its constituent herbs significantly stimulated the phosphorylation of extracellular signal-regulated kinases and cAMP-responsive element binding protein, two molecular targets involved in learning and memory, as well as in the regulation of neurogenesis.
Conclusions: Several constituents of YXT possess multiple regulatory effects on known therapeutic targets of AD that range from β-amyloid to acetylcholinesterase. The demonstrated neuroprotective and neurogenic actions of YXT lend credence to its use as an alternative medicine for treating AD.
Keywords: Alzheimer’s disease; Chinese medicine; Neural progenitor cell; Neurodegenerative disease; Neuroprotection; Yang Xin Tang; β amyloid.
© 2023. International Society for Chinese Medicine and BioMed Central Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Chen SY, Gao Y, Sun JY, Meng XL, Yang D, Fan LH, Li X, Wang P. Traditional Chinese medicine: role in reducing β-Amyloid, apoptosis, autophagy, neuroinflammation, oxidative stress, and mitochondrial dysfunction of Alzheimer’s disease. Front Pharmacol. 2020;11:497. doi: 10.3389/fphar.2020.00497. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

