Anti-PD-1/Her2 Bispecific Antibody IBI315 Enhances the Treatment Effect of Her2-Positive Gastric Cancer through Gasdermin B-Cleavage Induced Pyroptosis
- PMID: 37587833
- PMCID: PMC10602533
- DOI: 10.1002/advs.202303908
Anti-PD-1/Her2 Bispecific Antibody IBI315 Enhances the Treatment Effect of Her2-Positive Gastric Cancer through Gasdermin B-Cleavage Induced Pyroptosis
Abstract
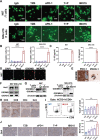
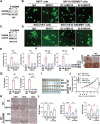
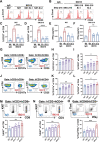
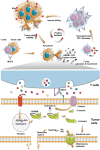
The majority of patients with human epidermal growth factor receptor 2 (Her2)-positive gastric cancer develop refractory to Her2-targeted therapy, where upregulation of immune checkpoints plays an essential role. Herein, a recombinant fully human IgG1 bispecific antibody IBI315 targeting both PD-1 and Her2 is developed and its antitumor efficacy as well as the underlying mechanism is investigated. IBI315 crosslinks the physical interaction between Her2-positive tumor cells and PD-1-positive T cells, resulting in significantly enhanced antitumor effects compared to each parent antibody or their combination, both in vitro and in vivo mouse tumor models reconstituted with human immune cells using patient-derived xenografts and organoids. Moreover, IBI315 treatment also induces the recruitment and activation of immune cells in tumors. Mechanistically, IBI315 triggers gasdermin B (GSDMB)-mediated pyroptosis in tumor cells, leading to the activation and recruiments of T cells. The activated T cells secret IFNγ, enhancing GSDMB expression and establishing a positive feedback loop of T cell activation and tumor cell killing. Notably, GSDMB is found to be elevated in Her2-positive gastric cancer cells, providing a rationale for IBI315's efficacy. IBI315 is supported here as a promising bispecific antibody-based immunotherapy approach for Her2-positive gastric cancer in preclinical studies, broadening the therapeutic landscape of this patient population.
Keywords: GSDMB; Her2; bispecific antibodies; immunotherapy; pyroptosis.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
M.Z., J.X., W.W., and B.C. are employees of Innovent Biologics (Suzhou). All other authors declare no potential conflicts of interest.
Figures


References
-
- Bang Y. J., Van Cutsem E., Feyereislova A., Chung H. C., Shen L., Sawaki A., Lordick F., Ohtsu A., Omuro Y., Satoh T., Aprile G., Kulikov E., Hill J., Lehle M., Rüschoff J., Kang Y.‐K., Lancet 2010, 376, 687. - PubMed
-
- Hurvitz S. A., Martin M., Symmans W. F., Jung K. H., Huang C. S., Thompson A. M., Harbeck N., Valero V., Stroyakovskiy D., Wildiers H., Campone M., Boileau J. F., Beckmann M. W., Afenjar K., Fresco R., Helms H. J., Xu J., Lin Y. G., Sparano J., Slamon D., Lancet Oncol. 2018, 19, 115. - PubMed
-
- Valabrega G., Montemurro F., Sarotto I., Petrelli A., Rubini P., Tacchetti C., Aglietta M., Comoglio P. M., Giordano S., Oncogene 2005, 24, 3002. - PubMed
-
- Valabrega G., Montemurro F., Aglietta M., Ann. Oncol. 2007, 18, 977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
