The effects of tislelizumab treatment on the health-related quality of life of patients with advanced non-small cell lung cancer
- PMID: 37587845
- PMCID: PMC10501279
- DOI: 10.1002/cam4.6361
The effects of tislelizumab treatment on the health-related quality of life of patients with advanced non-small cell lung cancer
Abstract
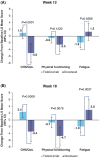
This study examined the health-related quality of life (HRQoL) of patients with advanced non-small cell lung cancer (NSCLC) receiving tislelizumab versus docetaxel in the open-label, multicenter, Phase 3 trial called RATIONALE-303 (NCT03358875). HRQoL was assessed with the EORTC QLQ-C30, EORTC QLQ-LC13, and the EQ-5D-5L instruments. A longitudinal analysis of covariance assessed the change from baseline to Week 12 and from baseline to Week 18. A time to deterioration analysis was also performed using the Kaplan-Meier method. Eight hundred and five patients were randomized to either tislelizumab (n = 535) or docetaxel, respectively (535 and 270 to tislelizumab and docetaxel, respectively). The tislelizumab arm improved while the docetaxel arm worsened in the QLQ-C30 global health status/QoL scale score (difference LS mean change Week 18: 5.7 [95% CI: 2.38, 9.07, p = 0.0008]), fatigue (Week 12: -3.2 [95% CI: -5.95, -0.37, p < 0.0266]; Week 18: -4.9 [95% CI: -8.26, -1.61, p = 0.0037]), and QLQ-LC13 symptom index score (Week 12: -5.5 [95% CI: -6.93, -4.04, P < 0.0001]; Week 18: -6.6 [95% CI: -8.25, -4.95, p < 0.0001]). The tislelizumab arm had improvements in coughing versus the docetaxel arm (Week 12: -4.7 [95% CI: -8.57, -0.78, p = 0.0188]; Week 18: -8.3 [95% CI: -13.02, -3.51, p = 0.0007]). The patients who received tislelizumab were less at risk for clinically meaningful worsening in the overall lung cancer symptom index scale (hazard ratio (HR): 0.24 [95% CI: 0.162, 0.356], p < 0.0001), dyspnea (HR: 0.74 [95% CI: 0.567, 0.958], p = 0.0109), coughing (HR: 0.74 [95% CI: 0.534, 1.019], p = 0.0309), and peripheral neuropathy (HR: 0.55 [95% CI: 0.370, 0.810] p = 0.0011). In general, tislelizumab versus docetaxel was associated with improved HRQoL and symptoms of lung cancer in patients who previously failed treatment with platinum-containing chemotherapy.
Keywords: docetaxel; health-related quality of life; non-small cell lung cancer; patient-reported outcomes; programmed cell death protein-1 inhibitor.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
GB, SL, LZ, and BT are employees of and own stock in BeiGene Ltd. DH reports no disclosures. YM is a former employee of Beigene and is now an employee of Boehringer International Ingelheim GmbH. CZ reports personal fees from Lily China, personal fees from BI, personal fees from Roche China, personal fees from MSD, personal fees from Qilu, personal fees from Hengrui, personal fees from Innovent Biologics, personal fees from C‐stone, personal fees from LUYE Pharma, personal fees from TopAlliance Biosciences Inc, personal fees from Amoy Diagnoistics, outside the submitted work.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

