Autophagy ablation in skeletal muscles worsens sepsis-induced muscle wasting, impairs whole-body metabolism, and decreases survival
- PMID: 37588163
- PMCID: PMC10425945
- DOI: 10.1016/j.isci.2023.107475
Autophagy ablation in skeletal muscles worsens sepsis-induced muscle wasting, impairs whole-body metabolism, and decreases survival
Abstract
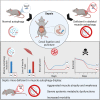
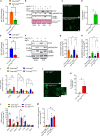
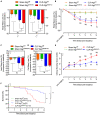
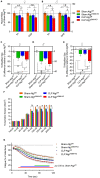
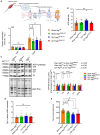
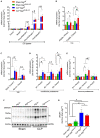
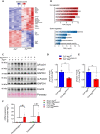
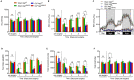
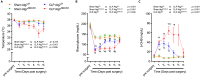
Septic patients frequently develop skeletal muscle wasting and weakness, resulting in severe clinical consequences and adverse outcomes. Sepsis triggers sustained induction of autophagy, a key cellular degradative pathway, in skeletal muscles. However, the impact of enhanced autophagy on sepsis-induced muscle dysfunction remains unclear. Using an inducible and muscle-specific Atg7 knockout mouse model (Atg7iSkM-KO), we investigated the functional importance of skeletal muscle autophagy in sepsis using the cecal ligation and puncture model. Atg7iSkM-KO mice exhibited a more severe phenotype in response to sepsis, marked by severe muscle wasting, hypoglycemia, higher ketone levels, and a decreased in survival as compared to mice with intact Atg7. Sepsis and Atg7 deletion resulted in the accumulation of mitochondrial dysfunction, although sepsis did not further worsen mitochondrial dysfunction in Atg7iSkM-KO mice. Overall, our study demonstrates that autophagy inactivation in skeletal muscles triggers significant worsening of sepsis-induced muscle and metabolic dysfunctions and negatively impacts survival.
Keywords: Genetics; Human metabolism; Musculoskeletal medicine.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Cheung A.M., Tansey C.M., Tomlinson G., Diaz-Granados N., Matté A., Barr A., Mehta S., Mazer C.D., Guest C.B., Stewart T.E., et al. Two-Year Outcomes, Health Care Use, and Costs of Survivors of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2006;174:538–544. doi: 10.1164/rccm.200505-693OC. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

