Transferrin-Conjugated PLGA Nanoparticles for Co-Delivery of Temozolomide and Bortezomib to Glioblastoma Cells
- PMID: 37588263
- PMCID: PMC10426337
- DOI: 10.1021/acsanm.3c02122
Transferrin-Conjugated PLGA Nanoparticles for Co-Delivery of Temozolomide and Bortezomib to Glioblastoma Cells
Abstract
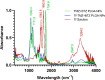
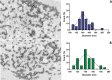
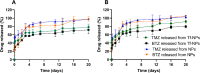
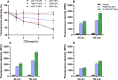
Glioblastoma (GBM) represents almost half of primary brain tumors, and its standard treatment with the alkylating agent temozolomide (TMZ) is not curative. Treatment failure is partially related to intrinsic resistance mechanisms mediated by the O6-methylguanine-DNA methyltransferase (MGMT) protein, frequently overexpressed in GBM patients. Clinical trials have shown that the anticancer agent bortezomib (BTZ) can increase TMZ's therapeutic efficacy in GBM patients by downregulating MGMT expression. However, the clinical application of this therapeutic strategy has been stalled due to the high toxicity of the combined therapy. The co-delivery of TMZ and BTZ through nanoparticles (NPs) of poly(lactic-co-glycolic acid) (PLGA) is proposed in this work, aiming to explore their synergistic effect while decreasing the drug's toxicity. The developed NPs were optimized by central composite design (CCD), then further conjugated with transferrin (Tf) to enhance their GBM targeting ability by targeting the blood-brain barrier (BBB) and the cancer cells. The obtained NPs exhibited suitable GBM cell delivery features (sizes lower than 200 nm, low polydispersity, and negative surface charge) and a controlled and sustained release for 20 days. The uptake and antiproliferative effect of the developed NPs were evaluated in in vitro human GBM models. The obtained results disclosed that the NPs are rapidly taken up by the GBM cells, promoting synergistic drug effects in inhibiting tumor cell survival and proliferation. This cytotoxicity was associated with significant cellular morphological changes. Additionally, the biocompatibility of unloaded NPs was evaluated in healthy brain cells, demonstrating the safety of the nanocarrier. These findings prove that co-delivery of BTZ and TMZ in Tf-conjugated PLGA NPs is a promising approach to treat GBM, overcoming the limitations of current therapeutic strategies, such as drug resistance and increased side effects.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Stupp R.; Dietrich P.-Y.; Kraljevic S. O.; Pica A.; Maillard I.; Maeder P.; Meuli R.; Janzer R.; Pizzolato G.; Miralbell R.; et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J. Clin. Oncol. 2002, 20 (5), 1375–1382. 10.1200/JCO.2002.20.5.1375. - DOI - PubMed
-
- Ramalho M. J.; Andrade S.; Coelho M. Á. N.; Loureiro J. A.; Pereira M. C. Biophysical interaction of temozolomide and its active metabolite with biomembrane models: The relevance of drug-membrane interaction for Glioblastoma Multiforme therapy. European Journal of Pharmaceutics Biopharmaceutics 2019, 136, 156–163. 10.1016/j.ejpb.2019.01.015. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
