Safety of general anaesthetics on the developing brain: are we there yet?
- PMID: 37588272
- PMCID: PMC10430845
- DOI: 10.1016/j.bjao.2022.100012
Safety of general anaesthetics on the developing brain: are we there yet?
Abstract
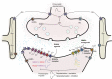
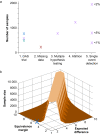
Thirty years ago, neurotoxicity induced by general anaesthetics in the developing brain of rodents was observed. In both laboratory-based and clinical studies, many conflicting results have been published over the years, with initial data confirming both histopathological and neurodevelopmental deleterious effects after exposure to general anaesthetics. In more recent years, animal studies using non-human primates and new human cohorts have identified some specific deleterious effects on neurocognition. A clearer pattern of neurotoxicity seems connected to exposure to repeated general anaesthesia. The biochemistry involved in this neurotoxicity has been explored, showing differential effects of anaesthetic drugs between the developing and developed brains. In this narrative review, we start with a comprehensive description of the initial concerning results that led to recommend that any non-essential surgery should be postponed after the age of 3 yr and that research into this subject should be stepped up. We then focus on the neurophysiology of the developing brain under general anaesthesia, explore the biochemistry of the observed neurotoxicity, before summarising the main scientific and clinical reports investigating this issue. We finally discuss the GAS trial, the importance of its results, and some potential limitations that should not undermine their clinical relevance. We finally suggest some key points that could be shared with parents, and a potential research path to investigate the biochemical effects of general anaesthesia, opening up perspectives to understand the neurocognitive effects of repetitive exposures, especially in at-risk children.
Keywords: anaesthetic drugs; apoptosis; biochemical mechanisms; duration of exposure; neuro-developmental risks; paediatric anaesthesia; single and multiple exposures.
© 2022 The Authors.
Figures

References
-
- Ikonomidou C., Bosch F., Miksa M., et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. - PubMed
-
- Ishimaru M.J., Ikonomidou C., Tenkova T.I., et al. Distinguishing excitotoxic from apoptotic neurodegeneration in the developing rat brain. J Comp Neurol. 1999;408:461–476. - PubMed
-
- Olney J.W., Labruyere J., Wang G., Wozniak D.F., Price M.T., Sesma M.A. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. - PubMed
-
- Olney J.W., Labruyere J., Price M.T. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. - PubMed
-
- Ikonomidou C., Bittigau P., Ishimaru M.J., et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

