Antinociceptive properties of losmapimod in two acute pain models in rats: behavioural analysis, immunohistochemistry, dose response, and comparison with usual analgesic drugs
- PMID: 37588580
- PMCID: PMC10430813
- DOI: 10.1016/j.bjao.2022.100029
Antinociceptive properties of losmapimod in two acute pain models in rats: behavioural analysis, immunohistochemistry, dose response, and comparison with usual analgesic drugs
Abstract
Background: The p38 protein is a ubiquitous mitogen-activated protein kinase involved in the proinflammatory signalling pathway and in the pain response after various noxious stimuli. Many p38 inhibitors have been developed and shown to provide effective analgesia in animal models. They are, however, mainly administered intrathecally or intravenously. Our study aimed to evaluate losmapimod, a novel oral p38 inhibitor, in two murine acute pain models.
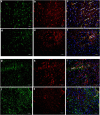
Methods: Losmapimod (12 mg kg-1) was compared with paracetamol, ketamine, and morphine using thermal and mechanical stimulation after carrageenan injection. A dose-effect study was also performed with this model. Behavioural testing was also performed in a plantar incision model to confirm the analgesic effect of losmapimod. Expression of activated p38 in neurones, microglia, and astrocytes was also investigated at 2, 15, and 24 h after carrageenan injection.
Results: Losmapimod was both antiallodynic and antihyperalgesic in the carrageenan pain model and provided an antinociceptive effect similar to that of morphine. The dose of 12 mg kg-1 was shown to be the ED78 and ED64 after thermal and mechanical stimulation, respectively. After plantar incision, losmapimod provided a significant antinociceptive effect. No life-threatening side-effect was observed in the behavioural study. Losmapimod prevented neurone and microglial activation at 2 and 15 h after carrageenan injection, respectively, but no effect was found on astrocytic activation.
Conclusion: Losmapimod appears to be a promising drug in severe acute pain conditions. Losmapimod could also be helpful for postoperative pain control, as suggested by its effect after plantar incision.
Keywords: acute pain; antinociceptive drug; losmapimod; p38 inhibition; rodent.
© 2022 The Author(s).
Figures




References
-
- Pearson G., Robinson F., Beers Gibson T., et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. - PubMed
-
- Kyriakis J.M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. - PubMed
-
- Kumar S., Boehm J., Lee J.C. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. - PubMed
-
- Cuadrado A., Nebreda A.R. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. - PubMed
LinkOut - more resources
Full Text Sources

